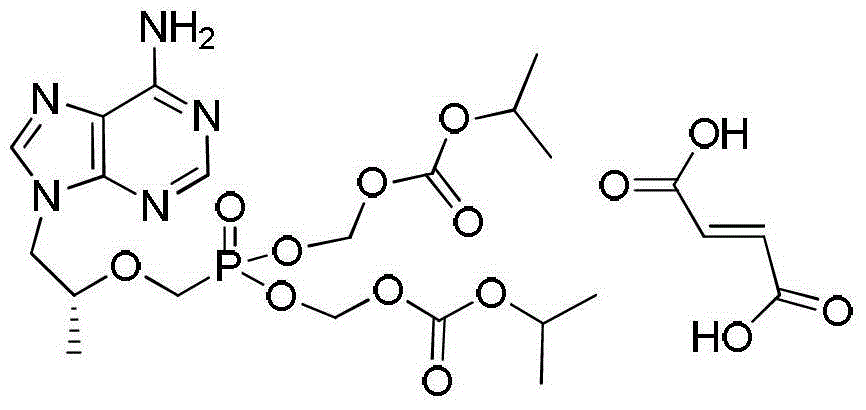
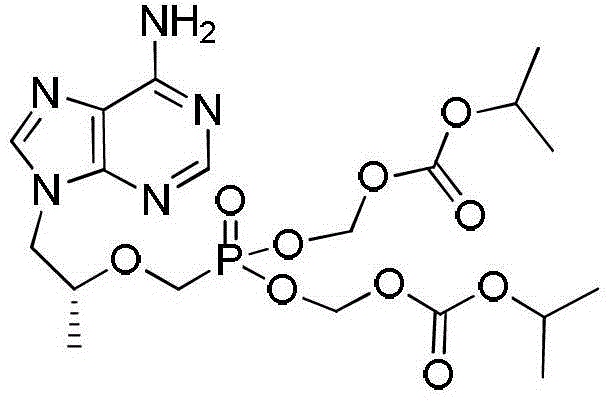
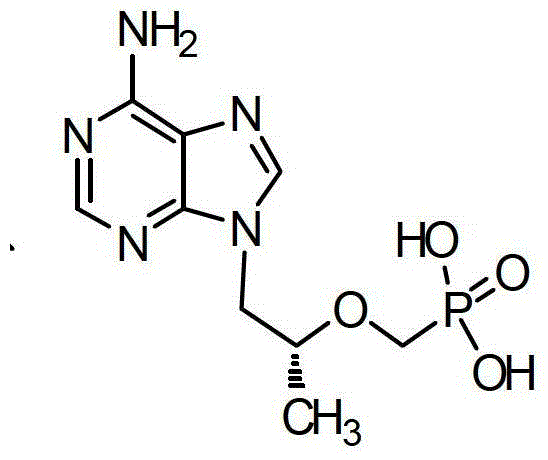
The preparation method of tenofovir disoproxil fumarate intermediate
A phosphoric acid and system technology, applied in the field of medicine, can solve problems such as difficulty in stirring, and achieve the effects of solving difficulty in stirring, realizing industrialized production and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of intermediate one
[0040] Add 10.00kg of starting materials and 40L of N-methylpyrrolidone into a 100L reactor, and slowly add 15kg of magnesium tert-butoxide under uniform stirring. Start heating, and start to add 20 kg of diethyl p-toluenesulfonyloxymethylphosphonate dropwise after the temperature rises to 70°C. After the addition is complete, keep the temperature at about 79°C for continuous reaction, and the basic reaction of the starting materials is detected by TLC. Stop heating and cool to room temperature naturally.
[0041]Add acetic acid to the system to adjust the pH to 7.0, and stir for 30 minutes to dissolve the system. Slowly add the reaction solution into 300L ethyl acetate, stir rapidly, a large amount of solids precipitate out, continue to stir for half an hour after the addition, and centrifuge. The filter cake was beaten with 50L of dichloromethane and centrifuged. After the filtrates were combined, they were concen...
Embodiment 2
[0042] Embodiment 2: the preparation of intermediate two
[0043] Add the N-methylpyrrolidone solution of intermediate 1 into a 100L reaction kettle, add 18.5kg of bromotrimethylsilane dropwise with stirring, and finish the dropwise addition in about one hour, then heat to 75°C, and stir to react. After the end point of the reaction was controlled by TLC to determine the completion of the raw materials, the temperature was lowered to quench the reaction.
[0044] Slowly add 30L of water to the system, then wash with 20L×2 ethyl acetate, adjust the pH of the water layer to 3 with 40% NaOH solution, and slowly cool the system to 0-5°C and slowly stir for crystallization. After crystallization for 3 hours, centrifugation, the obtained solid was washed with 4 L of ice water, and the obtained solid was air-dried at 70° C. for 8 hours to obtain 9.6 kg of crude product. After the crude product was recrystallized with water, it was filtered and dried to obtain 8.5 kg of the refined p...
Embodiment 3
[0045] Embodiment 3: the preparation of tenofovir disoproxil
[0046] Add 30L of N-methylpyrrolidone, 8.5kg of tenofovir, 8.9kg of triethylamine and 7.5kg of tetrabutylammonium bromide into the reaction kettle in sequence, and after stirring for 20 minutes, start to raise the temperature to 50°C. Then, 22.5kg chloromethyl isopropyl carbonate was added dropwise rapidly from the constant pressure dropping funnel, and the reaction was stirred. The reaction was monitored by TLC, and when the reaction of the starting material was basically complete, the temperature was lowered to quench the reaction.
[0047] After cooling down, add 12L*2 cyclohexane to the system, stir and wash for 20min, and discard the cyclohexane in the upper layer after static separation. The N-methylpyrrolidone solution in the lower layer was transferred to a reaction kettle, 60L of water and 40L of ethyl acetate were added, stirred for 30 minutes, and then separated into layers, and the aqueous layer was ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com