Biodegradable and sugar responsive Y type polymer drug delivery material and preparation
A sugar-responsive, polymer technology is applied in the field of polymer drug delivery materials and their preparation to achieve the effects of being beneficial to human metabolism, good biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) The raw material phenylboronic acid (8.0g, 65.6mmol) and trimethylolethane (7.9g, 67.5mmol) were dissolved in toluene (100mL) and refluxed at 140°C for 5h. After the reaction was completed, the reaction solution was concentrated, and then vacuum-dried to obtain compound II.
[0033]
[0034] 2) N 2 Under protection, the above compound II (10.0g, 54.4mmol) and triethylamine (4.9g, 54.24mmol) were dissolved in 40mL of dichloromethane, and added to the above solution in an ice bath (0-4°C) A dichloromethane solution of acryloyl chloride (5.4 g, 66.1 mmol) was slowly added dropwise and reacted for 11 hours; after the reaction was terminated, the reaction solution was crudely purified, then the reaction solution was concentrated, and separated and purified by column chromatography to obtain compound III; 1 H NMR (400MHz, CDCl 3 ), δ (ppm): 1.04 (s, 3H, -CH 3 ), δ (ppm): 3.86 (d, J=12Hz, 2H, -BOCH 2 (CCH 3 )), 4.06 (d, J=8Hz, 2H, -BO-CH 2 (CCH 2 CH 3 )), 5.85 (d...
example 2
[0047] 1) Same as step 1 of embodiment 1)
[0048] 2) Same as step 2 of Example 1)
[0049] 3) Same as Step 3 of Example 1)
[0050] 4) Same as Step 4 of Example 1)
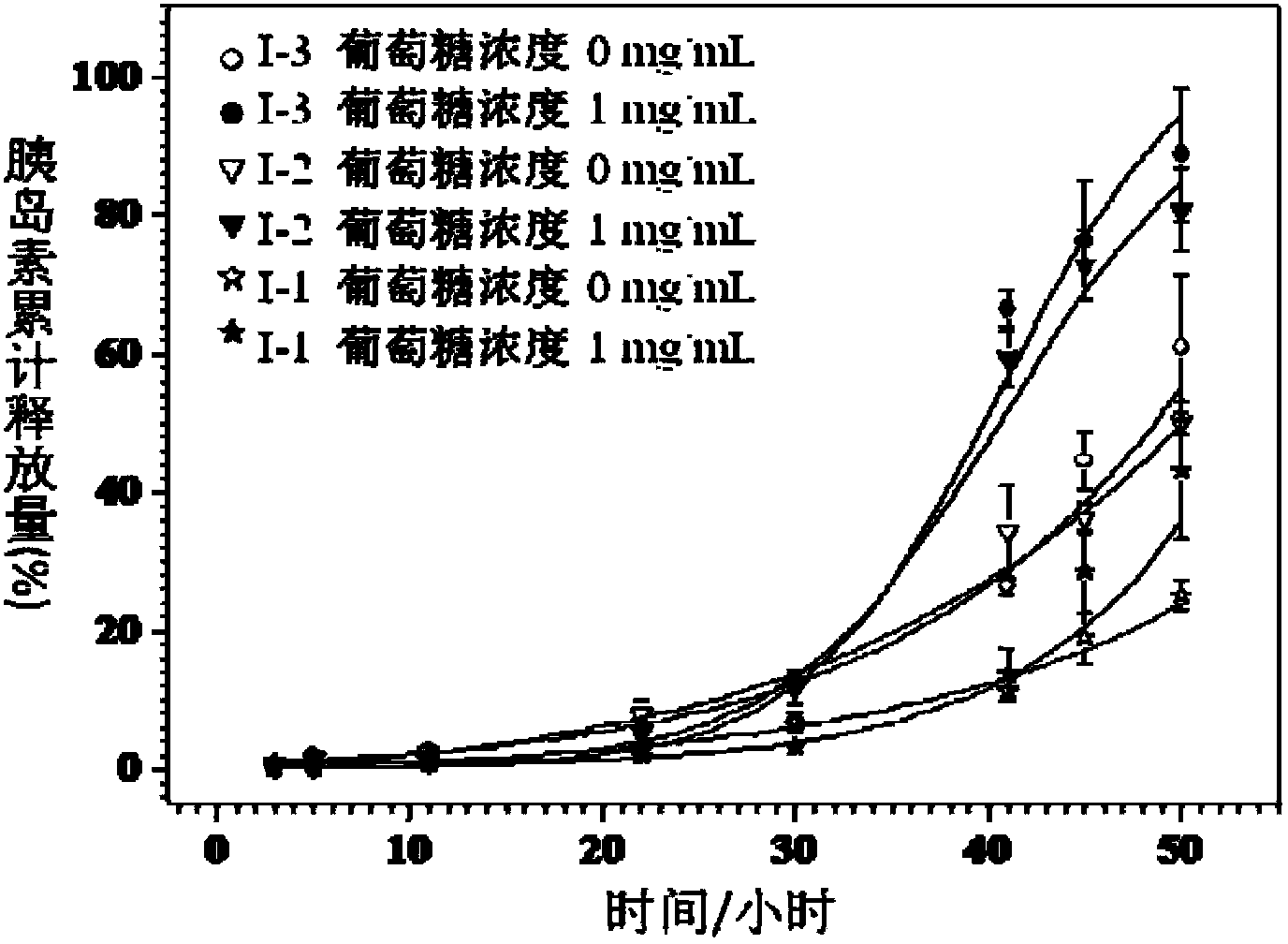
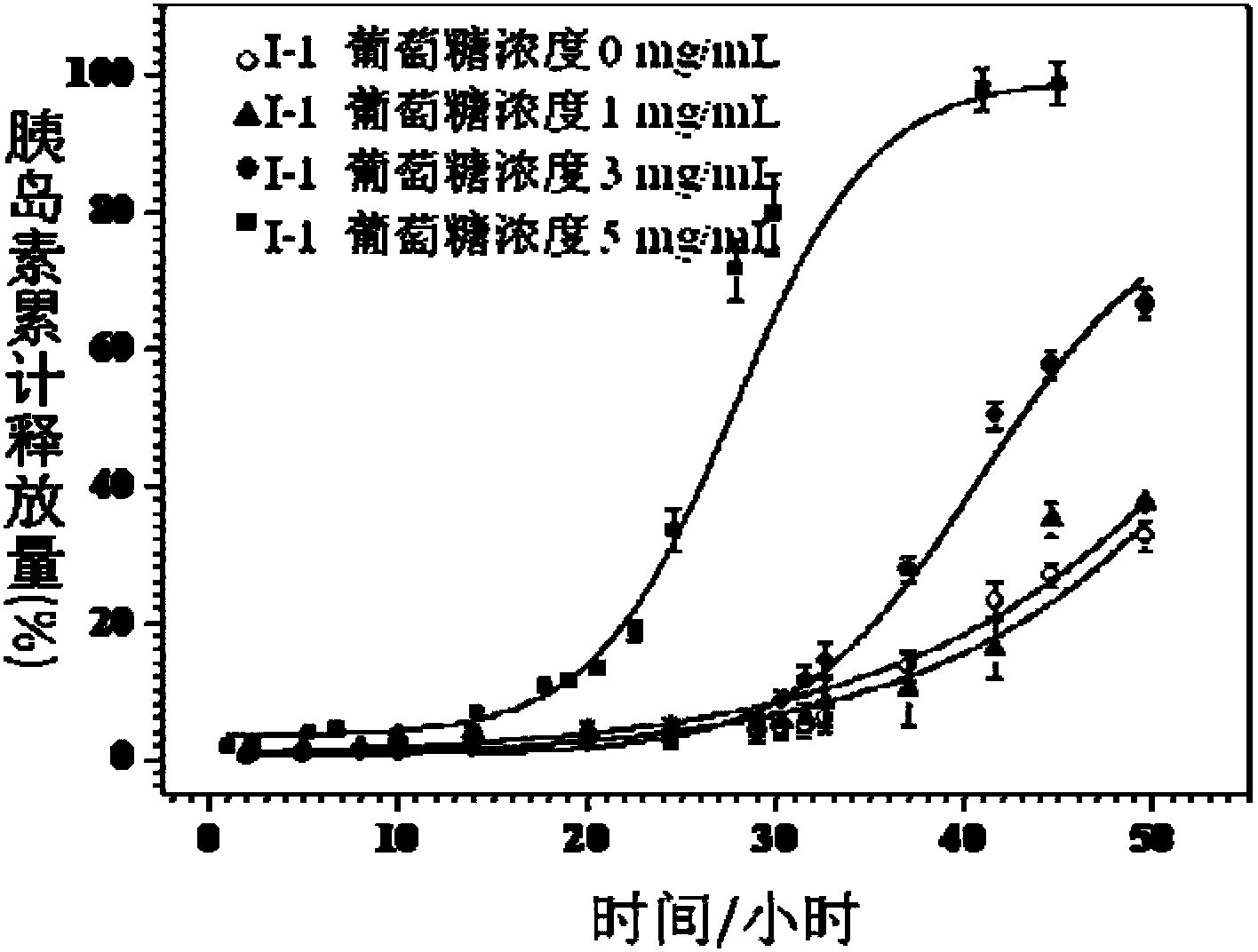
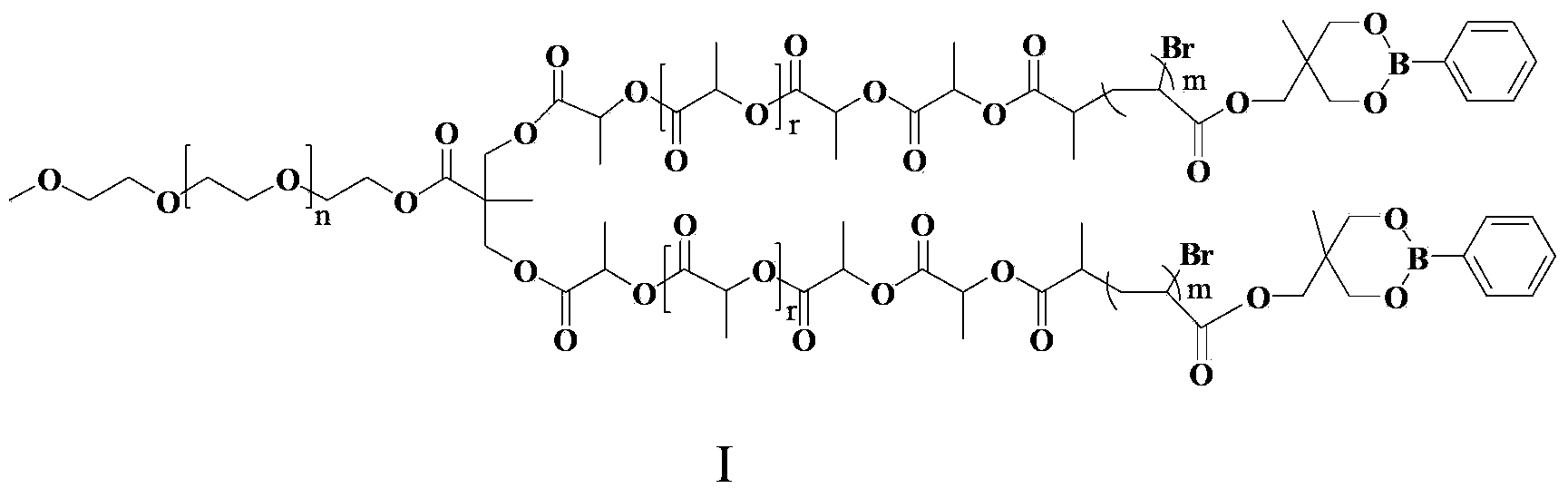
[0051] 5) in N 2 Under protection, add 250mg (0.05mmol) initiator V-1, 1.1mg (0.005mmol) copper bromide, 14mg (0.1mmol) cuprous bromide, 17.8mg (0.1mmol) pentylenediethylene to the reaction device Triamine and 2.0g (7.7mmol) of compound III were dissolved in 2.1mL of anisole and reacted for 18 hours under nitrogen protection at 90°C. The reaction solution was concentrated and precipitated in ether / n-hexane to obtain product I-2 .
[0052]
[0053] Where n=44, r=10, m=39
example 3
[0055] 1) Same as step 1 of embodiment 1)
[0056] 2) Same as step 2 of Example 1)
[0057] 3) Same as Step 3 of Example 1)
[0058] 4) Same as Step 4 of Example 1)
[0059] 5) in N 2 Under protection, add 250mg (0.05mmol) initiator V-1, 1.1mg (0.005mmol) copper bromide, 14mg (0.1mmol) cuprous bromide, 17.8mg (0.1mmol) pentylenediethylene to the reaction device Triamine and 1.5g (5.8mmol) of compound III were dissolved in 2.1mL of anisole and reacted for 18 hours under nitrogen protection at 90°C. The reaction solution was concentrated and precipitated in ether / n-hexane to obtain product I-3 .
[0060]
[0061] In the formula, n=44, r=10, m=25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com