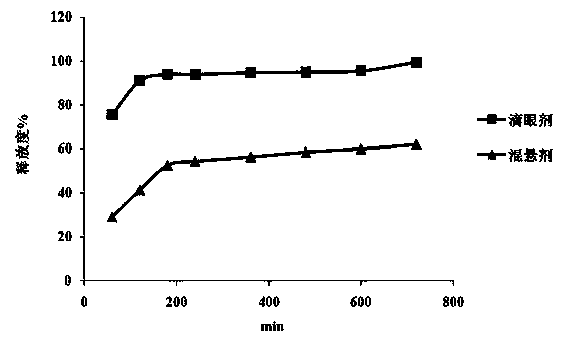
Sustained-release suspension for treating glaucoma and preparation method thereof
A slow-release suspension, glaucoma technology, applied in the field of medicine, can solve problems such as low bioavailability, and achieve the effects of drug safety, improved bioavailability and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The suspension is calculated by the following weight percentages: timolol maleate 1.5-2.5%, IRP-69 cation exchange resin 2.6-4.5%, microcapsule coating material 0.2-5%, plasticizer 6- 9%, impregnating agent 3.5-4%, suspending agent 9-17%, osmotic pressure regulator 0.2-4.5%, preservative 0.001-0.009%, pH regulator 0.2-5%, sorbitan monooleate 1.5% ~9%, liquid paraffin 11~20%, microcapsule coating material solvent 11~20% and ultrapure water 30~40%.
Embodiment 2
[0022] The suspension is calculated by the following percentages by weight: timolol maleate 3-4.5%, IRP-69 cation exchange resin 1.5-2.5%, microcapsule coating material 6-9%, plasticizer 0.2- 5%, impregnating agent 5-9%, suspending agent 0.2-8%, osmotic pressure regulator 5-9%, preservative 0.01-0.015%, pH regulator 6-9%, sorbitan monooleate 10% ~18%, liquid paraffin 5.5~10%, microcapsule coating material solvent 1.5~10% and ultrapure water 41~55%.
Embodiment 3
[0024] The suspension is calculated by the following weight percentages: timolol maleate 4%, IRP-69 cation exchange resin 2%, ethyl cellulose 2%, diethyl phthalate 5%, methyl Cellulose 3%, hydroxypropyl methylcellulose 5%, borax 3%, boric acid 3%, benzalkonium bromide 0.01%, triethylamine solution 5%, sorbitan monooleate 5%, liquid paraffin 20 %, absolute ethanol 10% and ultrapure water 32.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com