Method for preparing graphene based non-metallic oxygen reduction catalyst
A graphene-based, non-metallic technology, applied in graphene, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the limitations of fuel cells, metal-air battery commercialization, poor cycle stability of Pt-based catalysts, obstacles Battery performance improvement and other issues, to achieve the effects of aerobic reduction catalytic performance and stability, good oxygen reduction performance, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
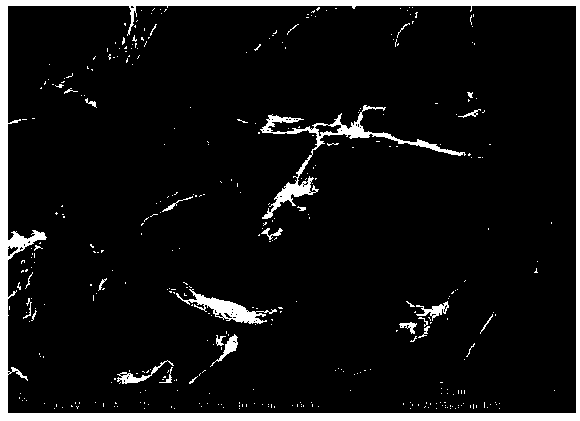
[0039] Graphene oxide was prepared according to Hummer's (Hummers, W.S. & Offeman, R.E. PREPARATION OF GRAPHITIC OXIDE. Journal of the American Chemical Society 80, 1339-1339 (1958)) method and formulated into a solution with a concentration of about 0.8 mg / ml. Take 10ml of the above solution and put it into a jar, place the jar in a water bath and stir vigorously with a magnetic stirrer, keeping the temperature at 90°C. After 2h, 50ml of sodium sulfide solution with a concentration of 20mg / ml was added dropwise, and stirring was continued at 90°C for 24h. The above solution is centrifuged, washed and dried to obtain the catalyst. The catalyst is in the form of flakes (see image 3 ), containing sulfur element, the content of sulfur element is 2.5mol%. Depend on Figure 5 It can be seen that there is an obvious oxygen reduction peak at about 0.45V, indicating that the prepared catalyst has oxygen reduction performance; Image 6It can be seen that after 1000 cycles, the pea...
Embodiment 2
[0041] Graphene oxide was prepared according to the method of hummer's (Hummers, W.S. & Offeman, R.E. PREPARATION OF GRAPHITIC OXIDE. Journal of the American Chemical Society 80, 1339-1339 (1958)) and formulated into a solution with a concentration of about 0.8 mg / ml. Take 10ml of the above solution and put it into a jar, place the jar in a water bath, and keep the temperature at 90°C. After 2h, 50ml of sodium sulfide solution with a concentration of 50mg / ml was added dropwise, and stirring was continued at 90°C for 24h. The above solution is centrifuged, washed and dried to obtain the catalyst. The catalyst is in block form (see Figure 4 ), containing sulfur element, the content of sulfur element is 1.2mol%. Depend on Figure 7 It can be seen that there is an obvious oxygen reduction peak at about 0.45V, indicating that the prepared catalyst has oxygen reduction performance; Figure 8 It can be seen that after 1000 cycles, the peak intensity and peak position of the oxyg...
Embodiment 3
[0043] Graphene oxide was prepared according to the method of hummer's (Hummers, W.S. & Offeman, R.E. PREPARATION OF GRAPHITIC OXIDE. Journal of the American Chemical Society 80, 1339-1339 (1958)) and formulated into a solution with a concentration of about 0.8 mg / ml. Take 10ml of the above solution and put it into a jar, place the jar in a water bath and stir vigorously with a magnetic stirrer, keeping the temperature at 70°C. After 2h, 20ml of 45wt% ammonium sulfide solution was added dropwise, and stirring was continued at 70°C for 24h. The above solution is centrifuged, washed and dried to obtain the catalyst. The catalyst is flake-shaped and contains sulfur and nitrogen elements. The content of sulfur element is 1.5mol%, and the content of nitrogen element is 2.1mol%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com