Preparation method of D-tryptophan lower alcohol ester hydrochloride with high optical purity
A technology of alcohol ester hydrochloride and alcohol ester sulfonate, which is applied in the field of preparation of tadalafil intermediates, can solve the problems of low optical purity and low yield, improve optical purity, stabilize the production process, avoid Effects of decomposition and other side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
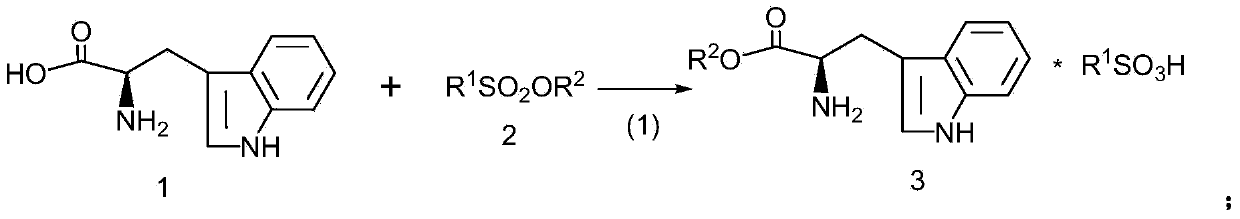
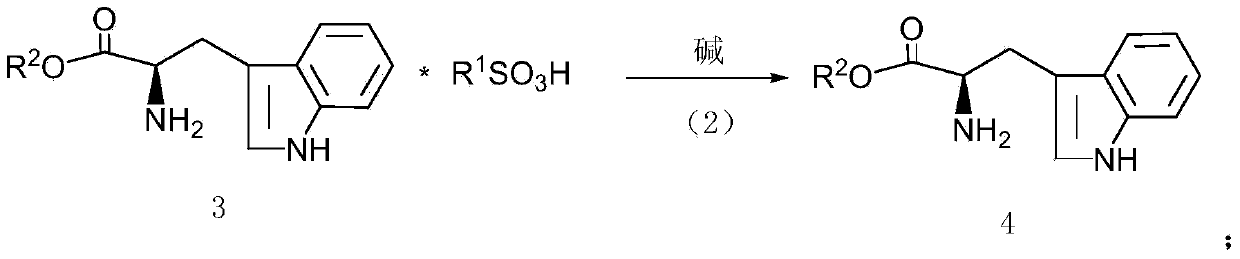
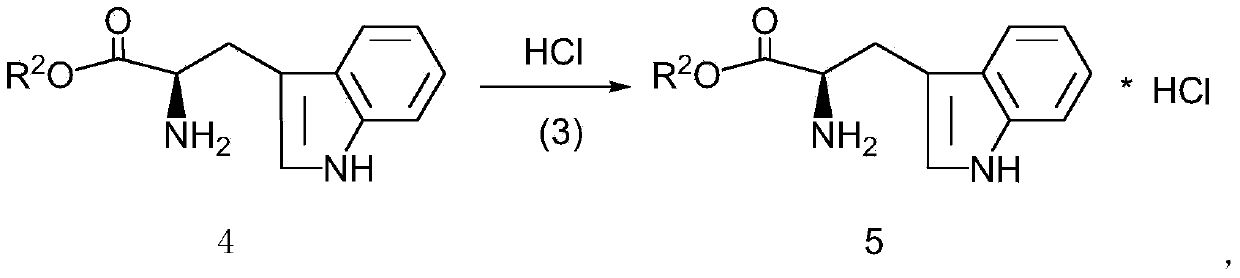
[0028] This example provides a kind of preparation method of D-tryptophan methyl ester hydrochloride, specifically as follows:
[0029] Add 1mol D-tryptophan (204g) into 400mL tetrahydrofuran, stir, add 206g 1.2mol methyl benzenesulfonate in batches, heat to 60°C, react for 8 hours, distill off tetrahydrofuran, add 300mL purified water, stir well Finally, adjust the pH to about 9.5 with 10wt% NaOH solution, extract with butyl acetate (250mL*3), combine the butyl acetate extract, wash with purified water (150mL*2), and dehydrate the butyl acetate extract with molecular sieve A3 overnight. Filter out the molecular sieves, pass dry hydrogen chloride gas into the butyl acetate extract, make the pH to about 4.5, evaporate most of the butyl acetate, and precipitate most of the crystals after cooling, and vacuum-dry the crystals after filtration to obtain a white solid 228 gram, which is D-tryptophan methyl ester hydrochloride, with an optical purity of 99.61% (HPLC), and a calculat...
Embodiment 2
[0031] This example provides a kind of preparation method of D-tryptophan ethyl ester hydrochloride, specifically as follows:
[0032] Add 1mol D-tryptophan (204g) into 400mL tetrahydrofuran, stir, add 223g 1.2mol ethyl benzenesulfonate in batches, heat to 60°C, react for 8 hours, distill off tetrahydrofuran, add 300mL purified water, stir well Finally, adjust the pH to about 9.0 with 10wt% NaOH solution, extract with butyl acetate (250mL*3), combine the butyl acetate extract, wash with purified water (150mL*2), and dehydrate the butyl acetate extract with molecular sieve A3 overnight. Filter out the molecular sieves, pass dry hydrogen chloride gas into the butyl acetate extract, make the pH to about 4.5, evaporate most of the butyl acetate, and precipitate most of the crystals after cooling, and vacuum-dry the crystals after filtration to obtain a white solid 236 gram, which is D-tryptophan ethyl ester hydrochloride, with an optical purity of 99.56% (HPLC), and a calculated ...
Embodiment 3
[0034] This example provides a kind of preparation method of D-tryptophan propyl ester hydrochloride, specifically as follows:
[0035] Add 1mol D-tryptophan (204g) into 400mL tetrahydrofuran, stir, add 240g 1.2mol propyl benzenesulfonate in batches, heat to 60°C, react for 8 hours, distill off tetrahydrofuran, add 300mL purified water, stir well Finally, adjust the pH to about 10 with 10wt% NaOH solution, extract with butyl acetate (250mL*3), combine the butyl acetate extract, wash with purified water (150mL*2), and dry the butyl acetate extract with anhydrous sodium sulfate , dehydrated overnight. Filter off anhydrous sodium sulfate, pass dry hydrogen chloride gas into the butyl acetate extract, make the pH to about 4.5, evaporate most of the butyl acetate, precipitate most of the crystals after cooling, and vacuum-dry the crystals after filtration to obtain 245 g of white solid is D-tryptophan propyl ester hydrochloride, the optical purity is 99.53% (HPLC), and the calcula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com