Preparation method of novel high nitrogen content compounds containing phosphaphenanthrene and phosphazene double-effect functional group
A compound and functionalization technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of no reports of phosphazenes and phosphaphenanthrenes with double-effect functional groups, and achieve The reaction system is green and environmentally friendly, the content of benzene ring is high, and the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
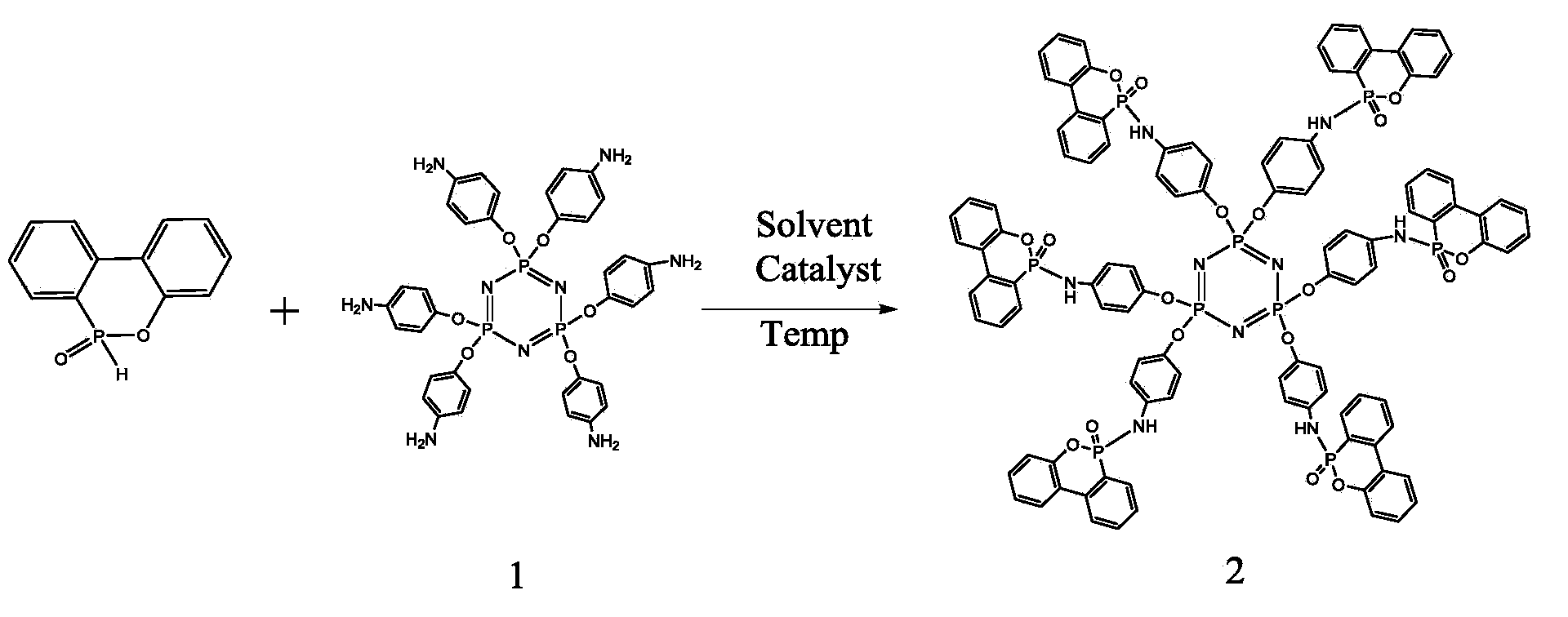
[0030] 6g DOPO, 2g hexaaminophenoxycyclotriphosphazene, 2g CCl 4 Dissolve in 100ml of methanol, heat to 80°C, and react for 10 hours; after the reaction is completed, cool the reaction to room temperature, filter the product with a Buchner funnel to obtain a yellow powder, add 50ml of acetone to wash 3 times, and obtain a light yellow powder. Dry in a vacuum oven to finally obtain product 2a, whose structural formula is as follows figure 1 Product 2, the product is 4.98g of powder, the yield is 96%, and the purity is 99.2%. 1 H NMR (DMSO-d6, TMS, ppm): 8.75 (br, 1H; NH), 31 P NMR (DMSO-d6, ppm): 8.0 (P-O), 8.9 (P=N)
example 2
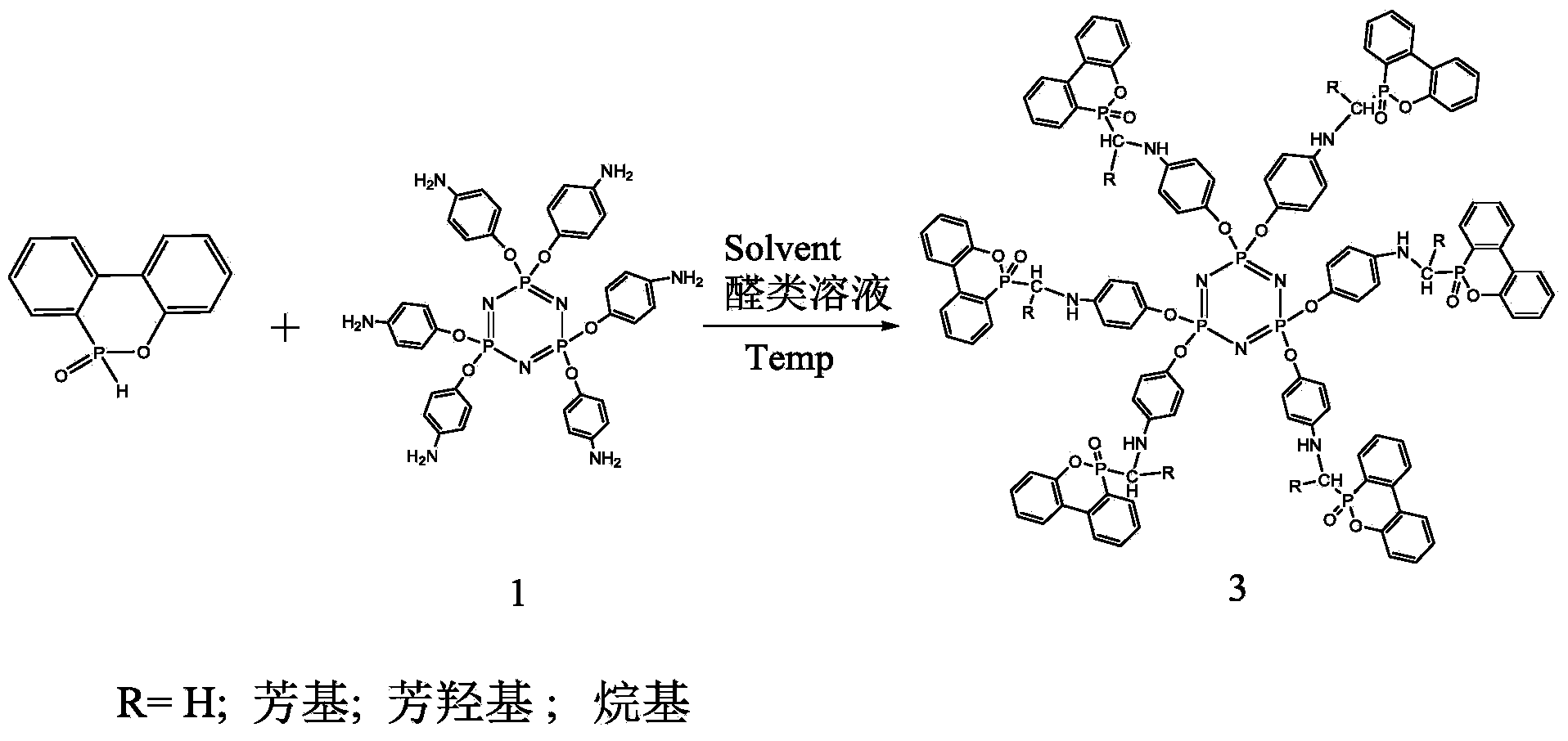
[0032] Dissolve 1.2g of hexaaminophenoxycyclotriphosphazene, 0.46g of formaldehyde solution and 2g of DOPO in 50ml of methanol, heat to 50°C, add 1g of AlCl after 1 hour of reaction 3 After the reaction is completed, the reaction is cooled to room temperature, and the product is filtered with a Buchner funnel to obtain a light yellow powder, and 50 ml of acetone is added to wash 3 times to obtain a white powder. when figure 2 When the R group in product 3 is H, the product is 2.99g of powder, the yield is 92%, and its nitrogen content is 5.86%. 1HNMR (DMSO-d6, TMS, ppm): 5.70 (s, 1H, NH), 4.02, 3.85 (m, 2H, CH 2 ), 31 P NMR (DMSO-d6, ppm): 32.11 (P-O), 9.75 (P=N)
example 3
[0034] Dissolve 2.4g of hexaaminophenoxycyclotriphosphazene, 1.2g of formaldehyde solution and 5g of DOPO in 100ml of acetone, heat to 90°C, and react for 10 hours; after the reaction is completed, the reaction is cooled to room temperature, and the product is filtered with a Buchner funnel to obtain Pale yellow powder, add 50ml of methanol and wash 3 times to obtain white powder, dry the product in a vacuum oven at 80°C, and finally obtain product 3a, whose structural formula is figure 2 When the R group in product 3 is H, the product is 6.1 g of powder, the yield is 94%, and its nitrogen content is 5.86%. 1H NMR (DMSO-d6, TMS, ppm): 5.70 (s, 1H, NH), 4.02, 3.85 (m, 2H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com