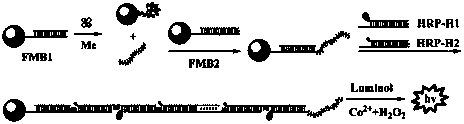
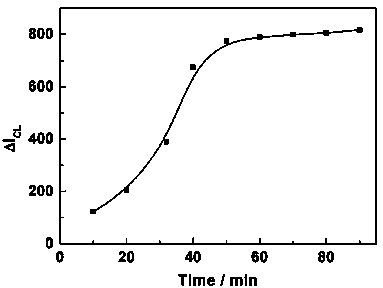
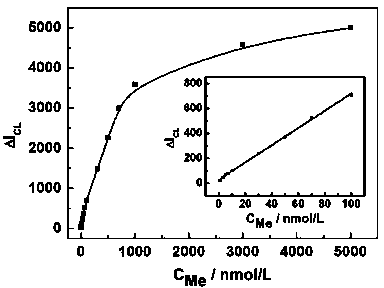
Method for determining melamine (Me) by chemiluminescence
A melamine and chemiluminescence technology, which is applied in the field of analytical chemistry and chemiluminescence sensors, can solve the problem of low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0030] Example: A method for the determination of melamine based on target-induced chain release and hybridization chain reaction chemiluminescence
[0031] 1. Experimental part
[0032] 1.1 Instruments and reagents
[0033] 1-(3-Dimethylaminoaldehyde)-3-ethyldiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Tianjin BASF Chemical Co., Ltd. Luminol (luminol) was purchased from Shanghai Aladdin Reagent Company. HRP (horseradish peroxidase) was purchased from Shanghai Shunshi Biotechnology Co., Ltd.
[0034] The artificially synthesized DNA sequence used was purchased from Beijing Saibaisheng Bioengineering Co., Ltd., and the sequence is as follows:
[0035] The partial sequence of DNA1 is: 5′-TTT TTT TTT TTT CCA AAA GGG-(CH 2 ) 6 -SH-3′
[0036] The partial sequence of DNA2 is: 5′-TGG AAA AAA AAA AAA-3′
[0037] The partial sequence of H1 is: 5'-NH 2 -(CH 2 ) 6 -GCG AGG TTT TTT TTT TTT CCA GCG CCG CAC AGA TGG AAA AAA AAA AAA-3′
[0038] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com