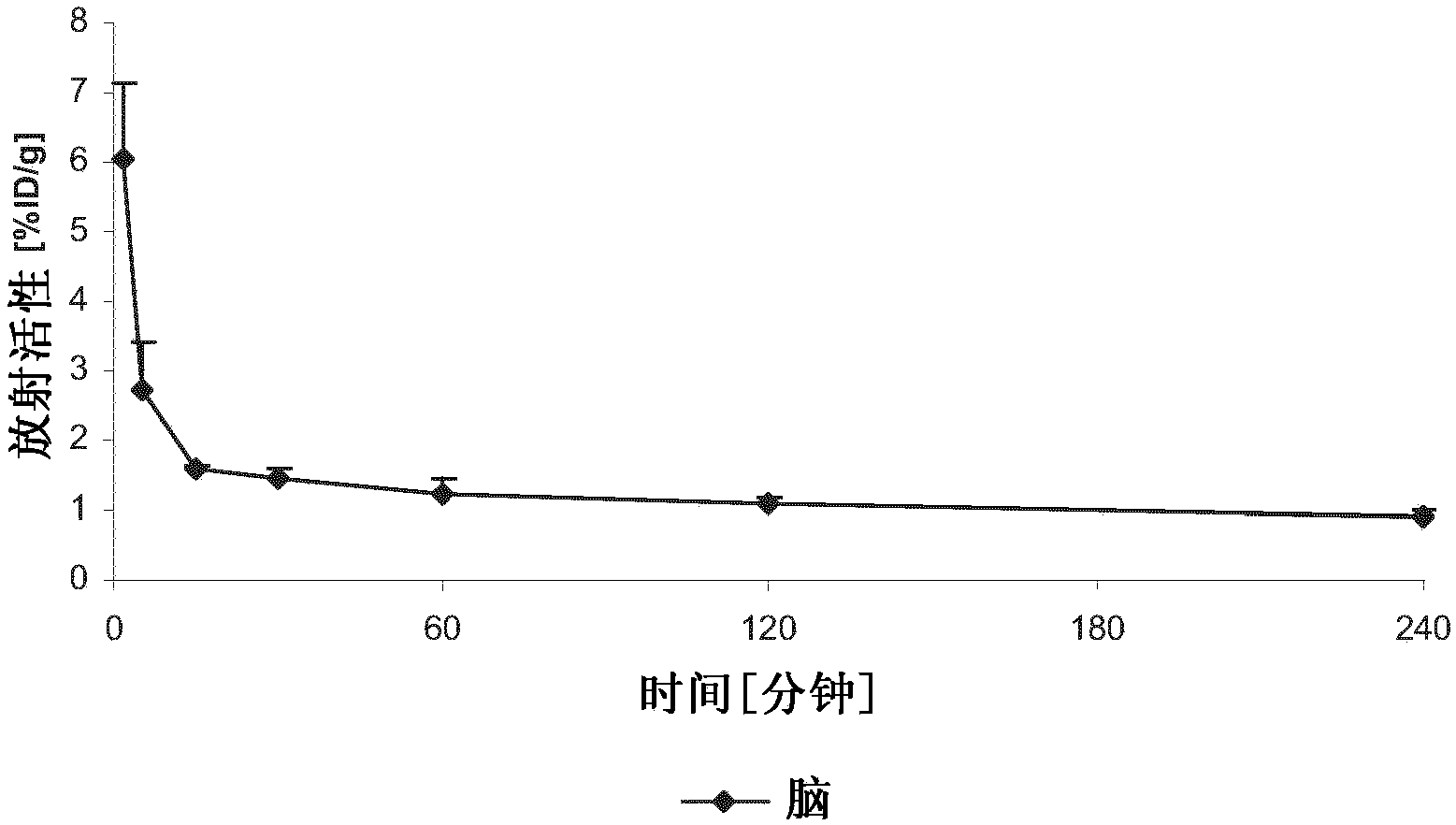
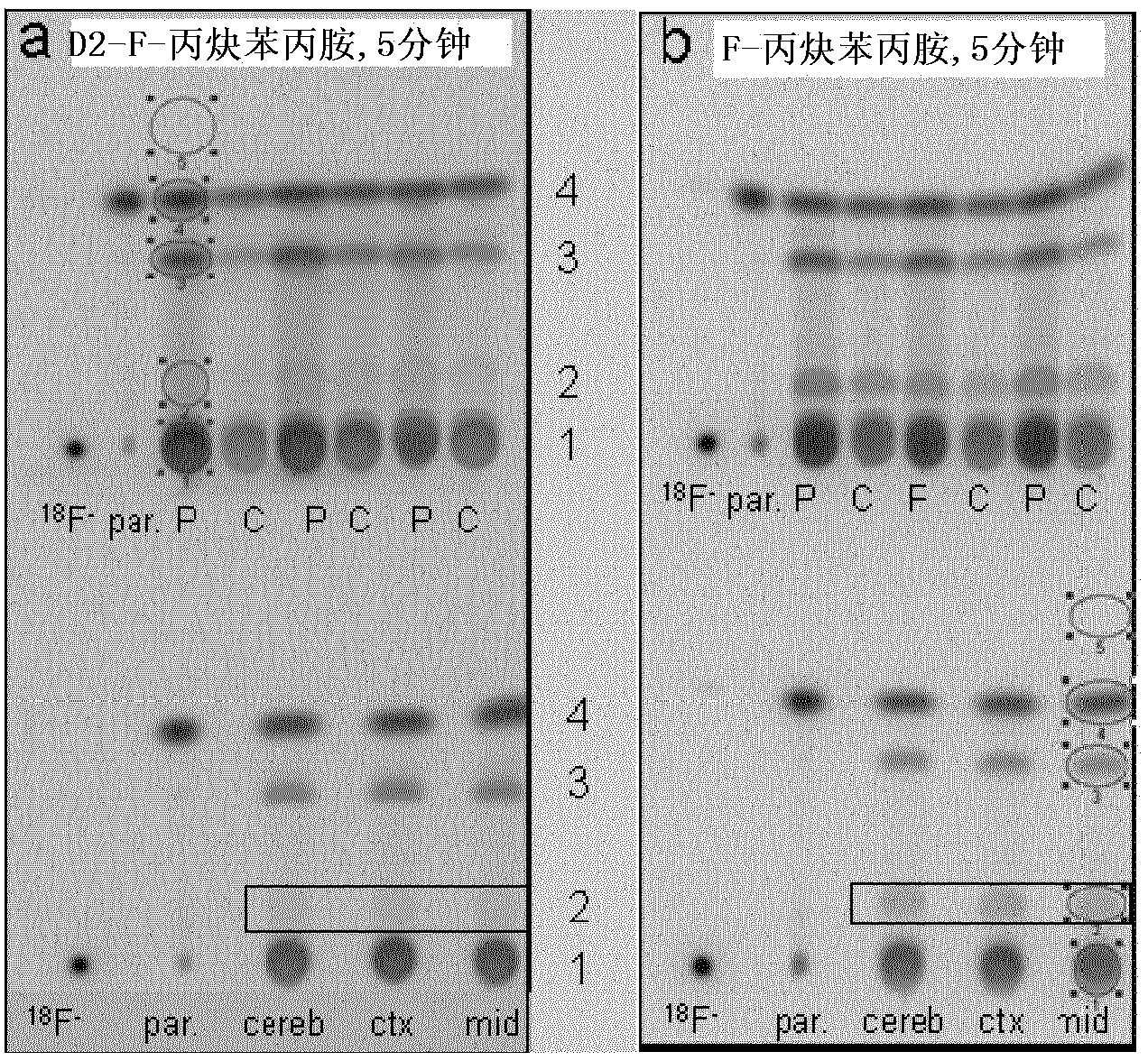
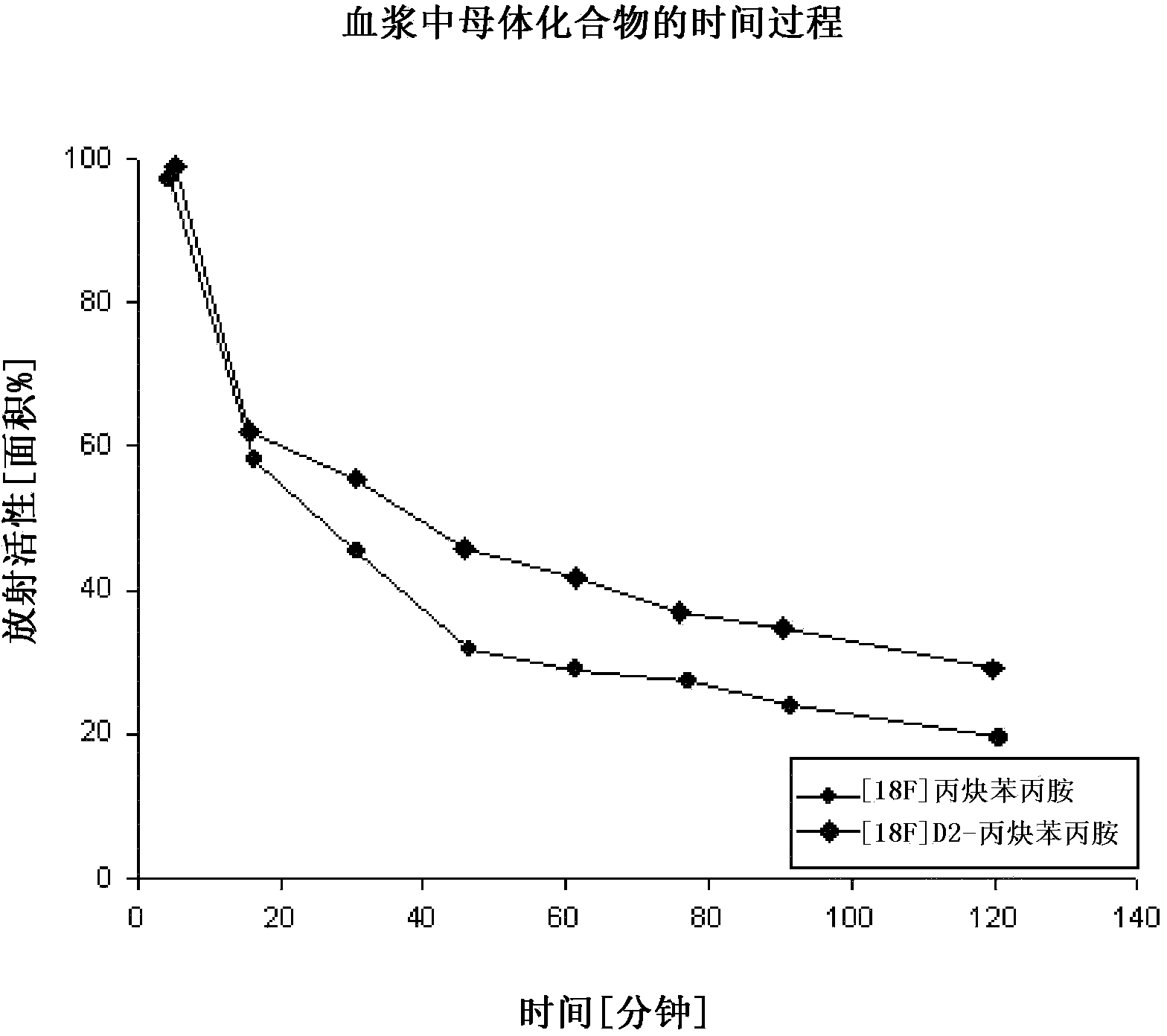
Compounds for use in imaging, diagnosing and/or treatment of diseases of the central nervous system
A compound and imaging technology, applied in the introduction of acyclic/carbocyclic compound isotopes, the preparation of amino compounds, the preparation of organic compounds, etc., can solve the problems of signal intensity reduction, capture rate reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] definition
[0038] As used herein, "leaving group" refers to a functional group selected from the group consisting of halogen, especially chlorine, bromine, iodine, methanesulfonyloxy, p-toluenesulfonyloxy, trifluoromethanesulfonyloxy Group, nonafluorobutanesulfonyloxy, (4-bromobenzene)sulfonyloxy, (4-nitrobenzene)sulfonyloxy, (2-nitrobenzene)-sulfonyloxy, (4-iso Propylbenzene)sulfonyloxy, (2,4,6-triisopropylbenzene)-sulfonyloxy, (2,4,6-trimethylbenzene)sulfonyloxy, (4-tert-butyl Benzene)sulfonyloxy, benzenesulfonyloxy and (4-methoxybenzene)sulfonyloxy.
[0039] When used herein by itself or as part of another group, the term "aryl" refers to a monocyclic ring containing 6 to 12 carbons in the ring portion, preferably 6-10 carbons in the ring portion Or a bicyclic aromatic group, such as phenyl, biphenyl, naphthyl or tetrahydronaphthyl. The preferred aryl group is phenyl.
[0040] When used herein in the specification and claims of the present invention, the term "alkyl"...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com