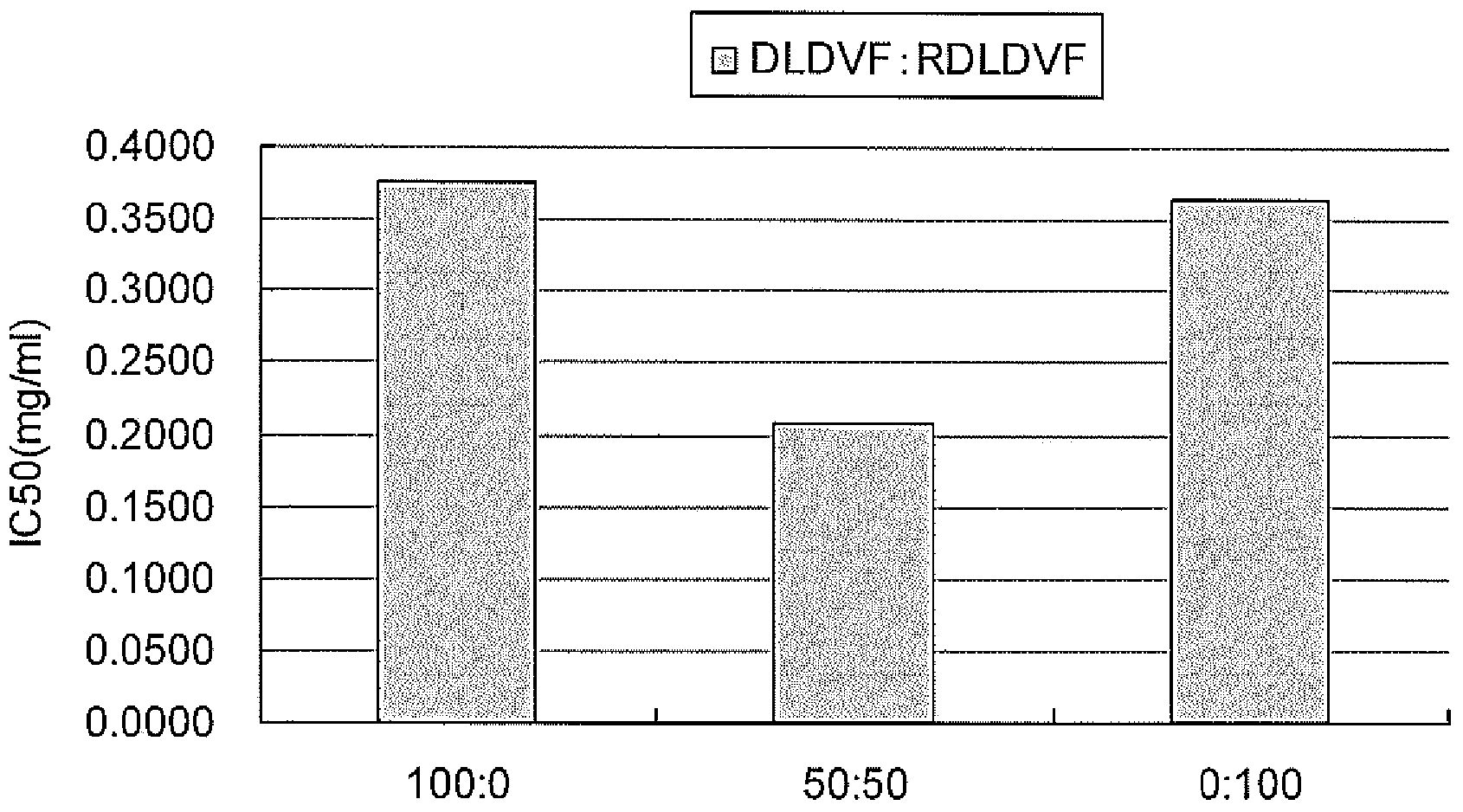Novel peptide
A technology of derivatives and compositions, applied in the field of new peptides or their derivatives or their salts, can solve the unknown problem of combining angiotensin-converting enzyme fibroblasts and collagen adhesion promotion activities to restore tension and elasticity , Solve beauty problems, improve wrinkles and sagging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the preparation of peptide
[0088] 1-1. DLDVF (Asp-Leu-Asp-Val-Phe) peptide:
[0089] (1) Synthesis of peptides:
[0090] The title peptide was synthesized by a solid-phase synthesis method using the Fmoc method using an automatic peptide synthesizer (manufactured by Shimadzu Corporation: PSSM8). The specific procedure is as follows: First, after the C-terminal of Fmoc-Phe(1-Trt)-OH is bound to the resin for solid-phase synthesis, the protective group (Fmoc) is removed by piperidine treatment, and then the resin is neutralized. · After washing, introduce Fmoc-Val-OH into the N-terminus of Phe. Next, the protective group (Fmoc) was removed by piperidine treatment, and after neutralizing and washing the resin again, Fmoc-Asp(OtBu)-OH was introduced into the N-terminal of Val. Next, the protective group (Fmoc) was removed by piperidine treatment, and after neutralizing and washing the resin again, Fmoc-Leu-OH was introduced into the N-terminal of Asp. N...
Embodiment 2
[0103] Example 2: Determination of angiotensin-converting enzyme inhibitory activity
[0104] The angiotensin-converting enzyme inhibitory activity was measured using the various peptides prepared in Example 1 above.
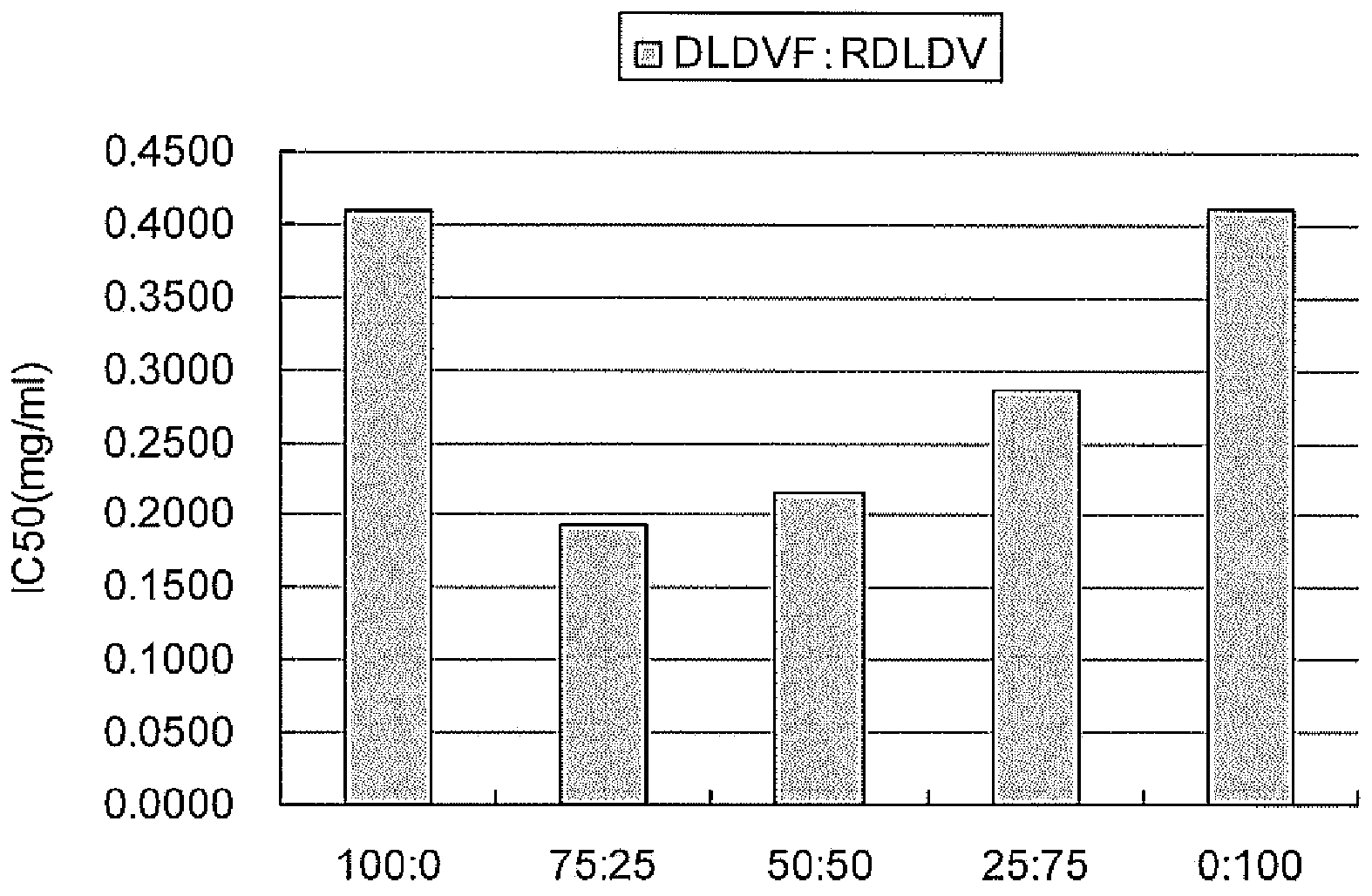
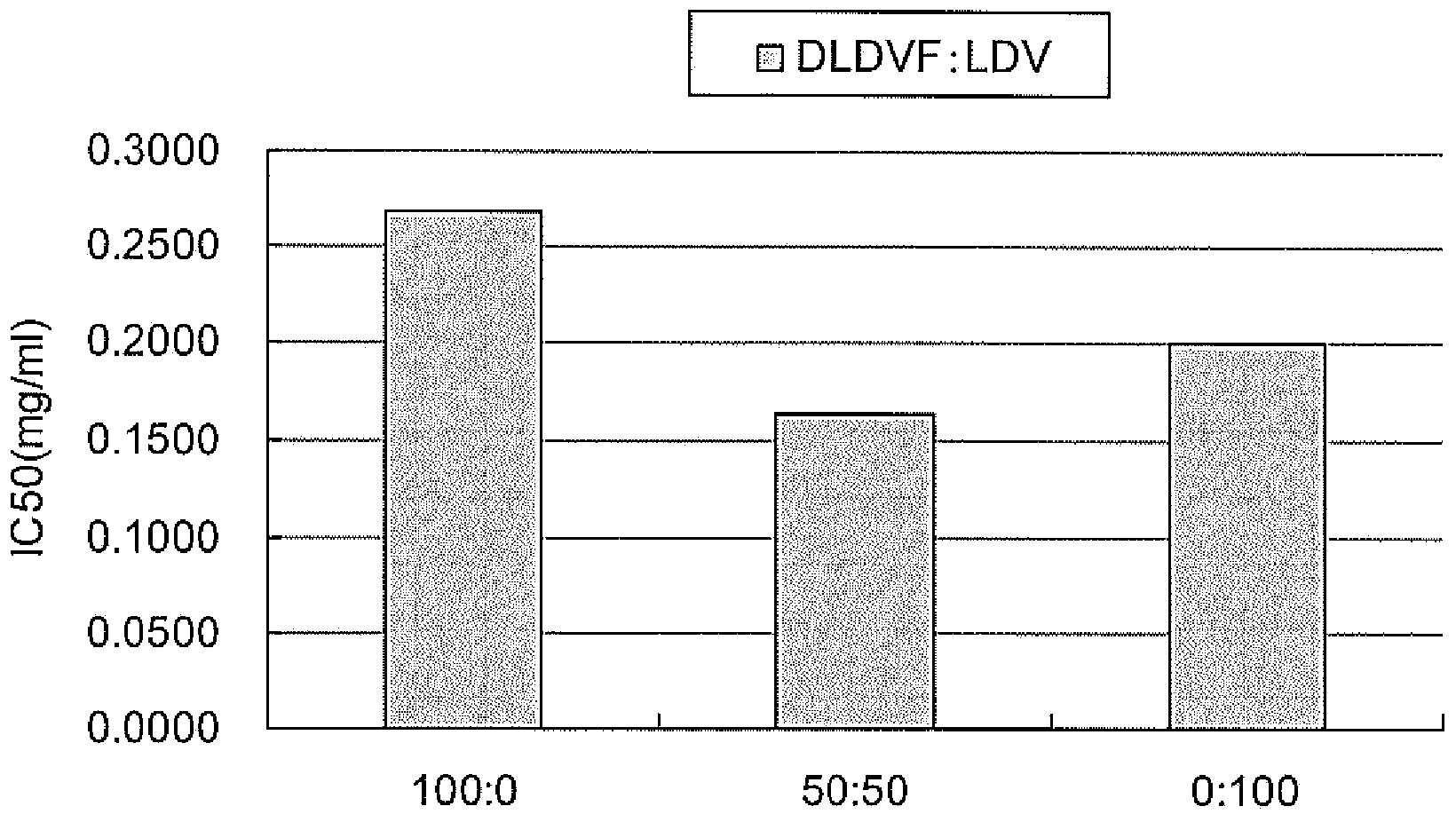
[0105] Specifically, test solutions were prepared so that the concentration of each peptide was 1 mg / mL, and 1-fold, 10-fold, 100-fold, and 1000-fold dilutions were used to measure angiotensin-converting enzyme inhibitory activity. The angiotensin-converting enzyme inhibitory activity was evaluated by using the ACE Kit-WST kit (manufactured by Dojin Chemical Research Institute) to detect 3-hydroxybutyryl-Gly-Gly-Gly (3HB-GGG ), and measure the absorbance at 450 nm with a microplate reader. The ACE Kit-WST kit was used according to the attached protocol. The results are shown below.
[0106] [Table 1]
[0107] peptide IC50(mg / mL) IC50(μM)
[0108] DLDV 0.4472 725.2 RDLDV 0.3275 428.7 RDLDVF 0.3018 395.1
[01...
Embodiment 3
[0110] Example 3: Evaluation of Fibroblast Adhesion to Collagen Coatings
[0111] The effect of promoting adhesion of fibroblasts to the collagen coating was evaluated using various peptides prepared in Example 1 above.
[0112] Specifically, first, 2 × 10 4 Fibroblasts (クラボウ: KF-4009) from human normal skin were inoculated at a concentration of cells / well. Next, 200 μl of Dulbecco’s modified Eagle’s medium (D-MEM; FCS10%·AB1%) diluted so that the concentration of each peptide was 5 μg / ml was added to each well. % carbon dioxide and 95% air environment for 20 hours of cultivation. On the other hand, cells to which 200 μl of D-MEM medium to which no peptide was added were prepared as a control. After 4 hours from the start of the culture, the supernatant of each well was washed with PBS, and the remaining amount of collagen coating adhered to and not washed was measured in each well using the Cell Counting Kit-8 kit (manufactured by Dojin Chemical Research Institute of Co., ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com