Technique for preparing monochlorodifluoromethane by decomposing trifluoromethane
A technology for difluoromonochloromethane and monofluorodichloromethane is applied in the field of trifluoromethane cracking to prepare difluoromonochloromethane, and achieves the effects of good economic value, less by-products, great operational feasibility and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
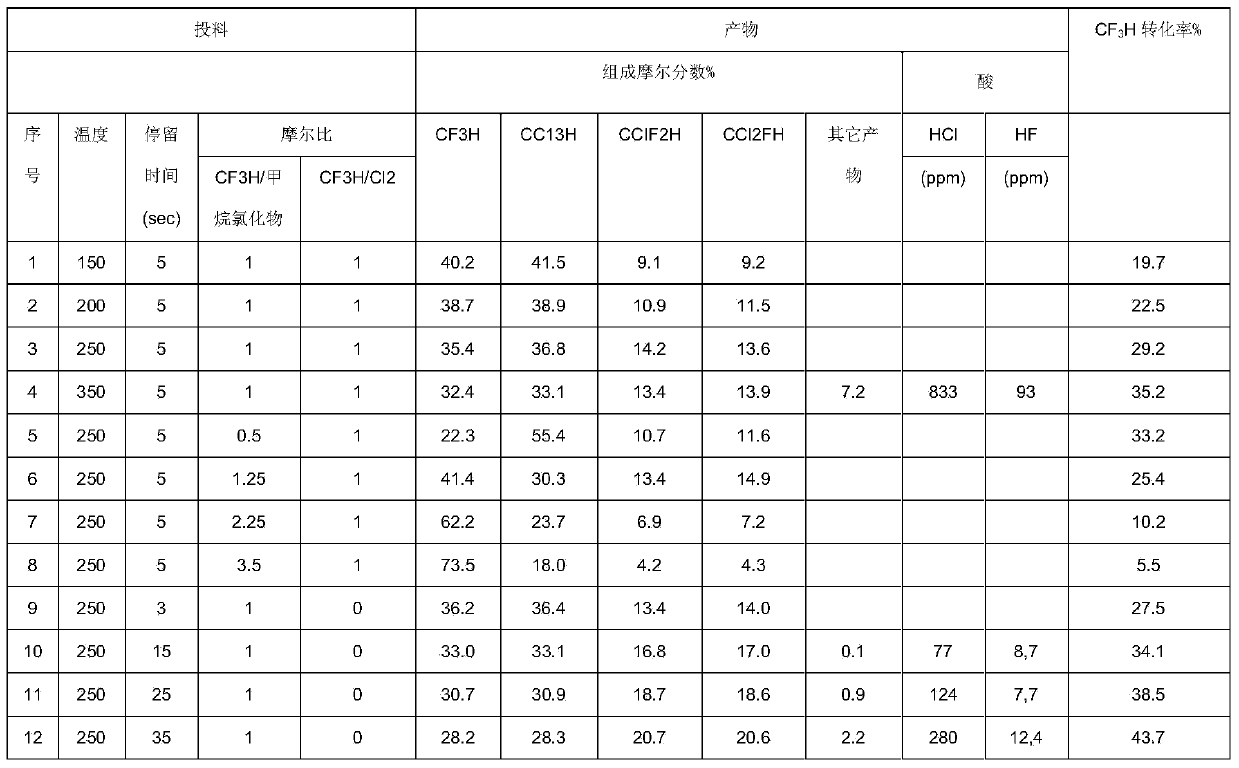
[0022] The catalyst is loaded into the nickel tube reactor and activated by passing hydrogen fluoride at 200°C for 6 hours. HFC-23, monochloromethane and chlorine gas were preheated to 120°C with a molar ratio of 1.0:1.0:1.0, respectively, and put into a nickel tube reactor at 150°C, with a residence time of 5s. A sample was taken one hour after feeding, and the mixed product was analyzed using GC / MS (Gas Chromatography / Mass Spectrometry) and the results obtained were: CF3H40.2%, CCl 3 H41.5%, CHF 2 Cl9.1%, CHCl 2 F9.2%.
Embodiment 2-4
[0024] The reaction temperatures of the nickel tubes are 200°C, 250°C, and 350°C respectively, and the rest of the conditions are the same as in Example 1. The mixed raw materials were put into the reaction and sampled after one hour, and the mixed products were analyzed by GC / MS (gas chromatography / mass spectrometry). The results obtained are shown in the table 1.
Embodiment 5-8
[0026] The molar ratios of HFC-23 and dichloromethane are 0.5, 1.25, 2.25, 3.5 respectively, and the molar number of chlorine is the same as that of HFC-23. Preheat to 120°C respectively, put them into a nickel tube reactor at 250°C, and keep The time is 5s. Samples were taken one hour after feeding, and the mixed product was analyzed by GC / MS (gas chromatography / mass spectrometry). The results obtained are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com