Method for separating alpha-glucosidase inhibitor from laver enzymolysis product
A technology of glucosidase and enzymatic hydrolysis products, which is applied in the field of separation and purification of biological products, can solve the problems of high price and scarce sources, achieve the effects of small molecular weight, prevent resource waste, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
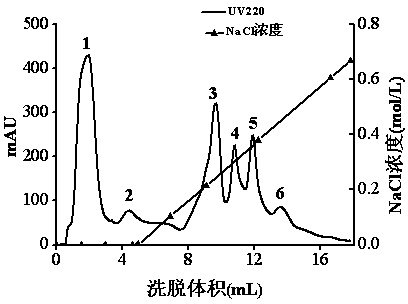
[0036] With 3 g of dried Porphyra striatus powder dried to constant weight as substrate, pH7.0 Na 2 HPO 4 -KH 2 PO 4 The buffer was used as the medium, the ratio of substrate to buffer was 1:33, and heat treatment was carried out in a boiling water bath for 15 min. Add neutral protease (E / S=8×10 4 U / g ), hydrolyzed at 50°C for 7 h, inactivated the enzyme in boiling water bath for 15 min; adjusted the pH to 8.5, added solid aminopeptidase ( E / S=28 U / g ) and alkaline protease ( E / S=8×10 4 U / g ), hydrolyze at 50°C for 3 h, inactivate the enzyme in a boiling water bath for 15 min, and add ethanol to a final concentration of 60%. Let it stand overnight at room temperature, centrifuge at 8000 r / min for 10 min, and the supernatant obtained is a mixture containing α-glucosidase inhibitor. The inhibitor-containing laver supernatant mixture was decolorized by activated carbon, and then separated and purified by 1 mL prepacked column SP Sepharose High Performance cation exchange c...
Embodiment 2
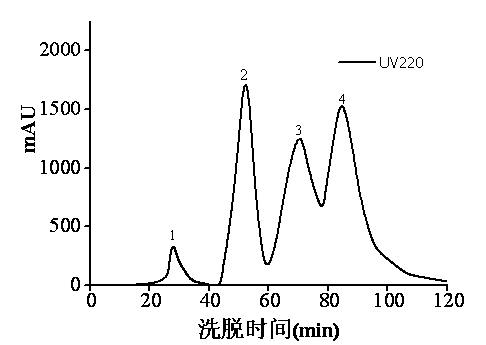
[0038] With 4 g of Porphyra zebra powder dried to constant weight as substrate, pH7.0 Na 2 HPO 4 -KH 2 PO 4 The buffer was used as the medium, the ratio of substrate to buffer was 1:25, and heat treatment was carried out in a boiling water bath for 15 min. Add neutral protease (E / S=8×10 4 U / g ), hydrolyzed at 50°C for 7 h, inactivated the enzyme in a boiling water bath for 15 min, adjusted the pH to 8.5, added solid aminopeptidase ( E / S=28 U / g ) and alkaline protease ( E / S=8×10 4 U / g ), hydrolyze at 50°C for 3 h, inactivate the enzyme in a boiling water bath for 15 min, and add ethanol to a final concentration of 60%. After standing overnight at room temperature, centrifuge at 8000 r / min for 10 min, and the obtained supernatant is a mixture containing inhibitors. The inhibitor-containing laver supernatant mixture was decolorized by activated carbon, and then separated and purified by 1 mL prepacked column SP Sepharose High Performance cation exchange chromatography. Eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com