Chiral catalyst in binaphthol synthesis technology
A technology of chiral catalyst and synthesis process, which is applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic compound preparation, etc. Industrial applicability, affecting product performance and other issues, to achieve the effect of simplifying recycling steps, low cost, and improving feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
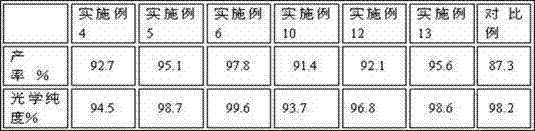
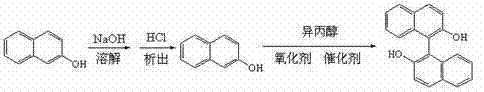
[0037] This example provides a preparation method of a chiral catalyst, which is realized through the following steps: dissolve 5.0g (1R,2R)1,2-cyclohexanediamine in 50mL of chloroform, ice bath, and then slowly drop 15.0g3-hydroxyl-2-naphthone, after reacting 12 hours, purify with silica gel column (mobile phase is : =1:1.5), to obtain 15.64g of yellow solid, dissolve the solid in 200mL of methanol, add 7.5g of ferric chloride, stir at room temperature for 3 hours, spin off the solvent, and recrystallize with n-hexane to obtain 19.14g of chiral catalyst a .
Embodiment 2
[0039] This example provides a preparation method of a chiral catalyst, which is realized through the following steps: dissolve 5.5g (1R,2R)1,2-cyclohexanediamine in 50mL of chloroform, ice-bath, and then slowly drop 18.0g3-hydroxyl-2-naphthone, after reacting for 18 hours, purify with silica gel column (mobile phase is : =1:1.5), to obtain 16.85 g of yellow solid, dissolve the solid in 250 mL of methanol, add 8.5 g of ferric chloride, stir at room temperature for 4.5 hours, spin off the solvent, and recrystallize with n-hexane to obtain 20.25 g of chiral catalyst b .
Embodiment 3
[0041] This example provides a preparation method of a chiral catalyst, which is realized by the following steps: dissolve 4.5g (1S,2S)1,2-cyclohexanediamine in 50mL of chloroform, ice-bath, and then slowly drop 14.0g3-hydroxyl-2-naphthone, reacted after 10 hours, purified with silica gel column (mobile phase is : =1:1.5), to obtain 13.57g of yellow solid, dissolve the solid in 180mL of methanol, add 7.0g of ferric chloride, stir at room temperature for 3 hours, spin off the solvent, and recrystallize with n-hexane to obtain 17.02g of chiral catalyst c .
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com