Method for preparing terminal carboxyl group polymer through active anionic polymer termination
A technology of active anions and active polymers, applied in the field of anionic polymerization, can solve the problems of less side reactions and harsh reaction conditions of side reactions, and achieve the effects of mild synthesis conditions, fast reaction speed and high functionalization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
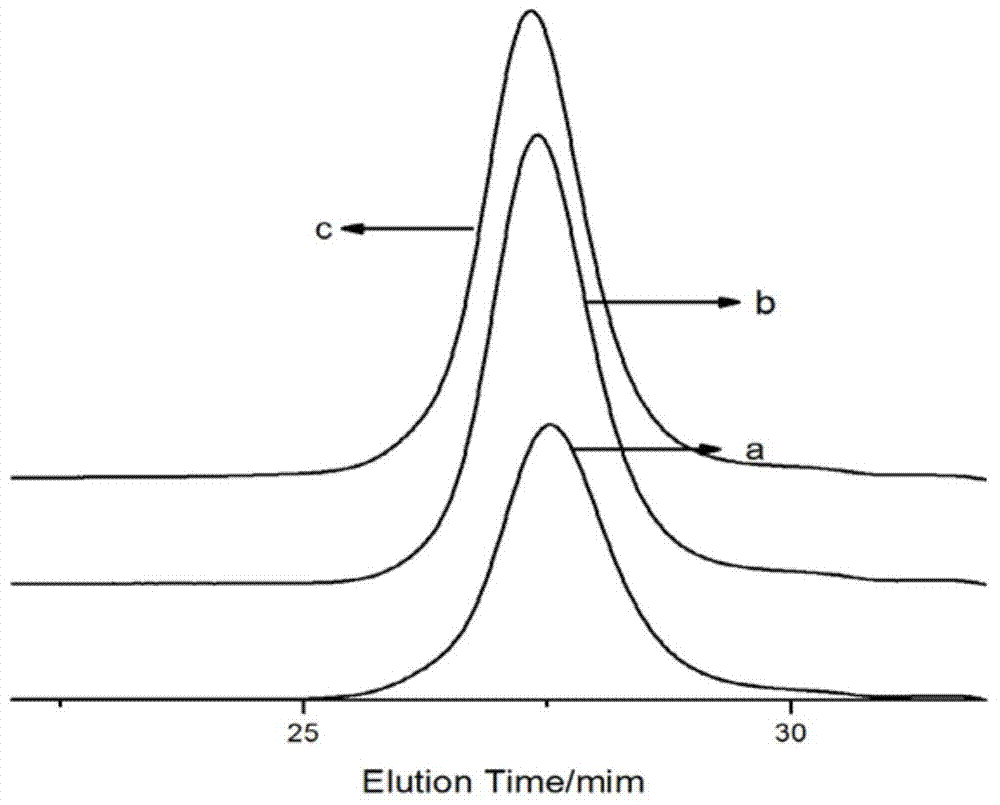
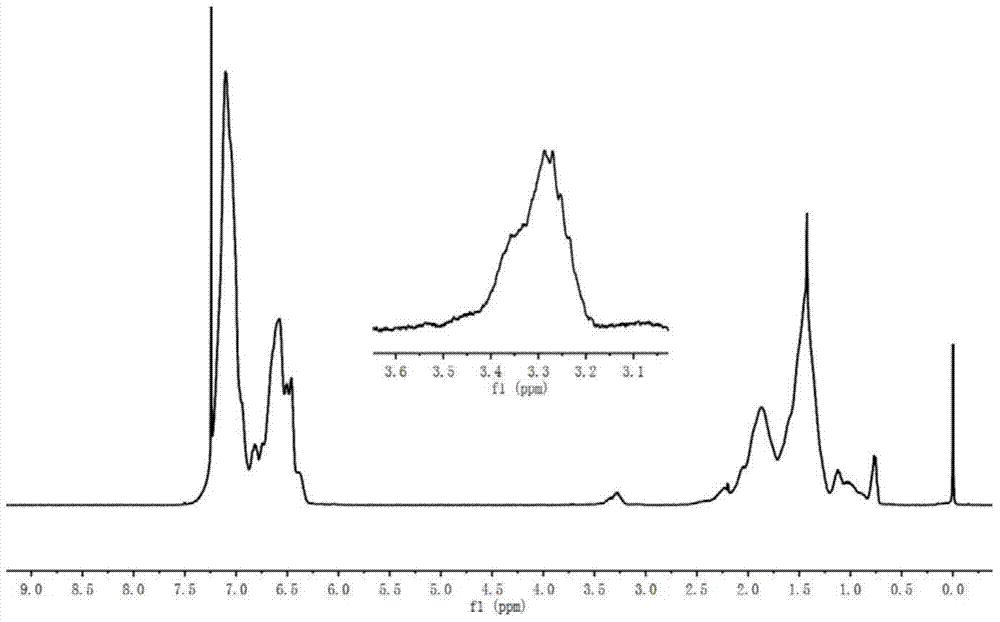
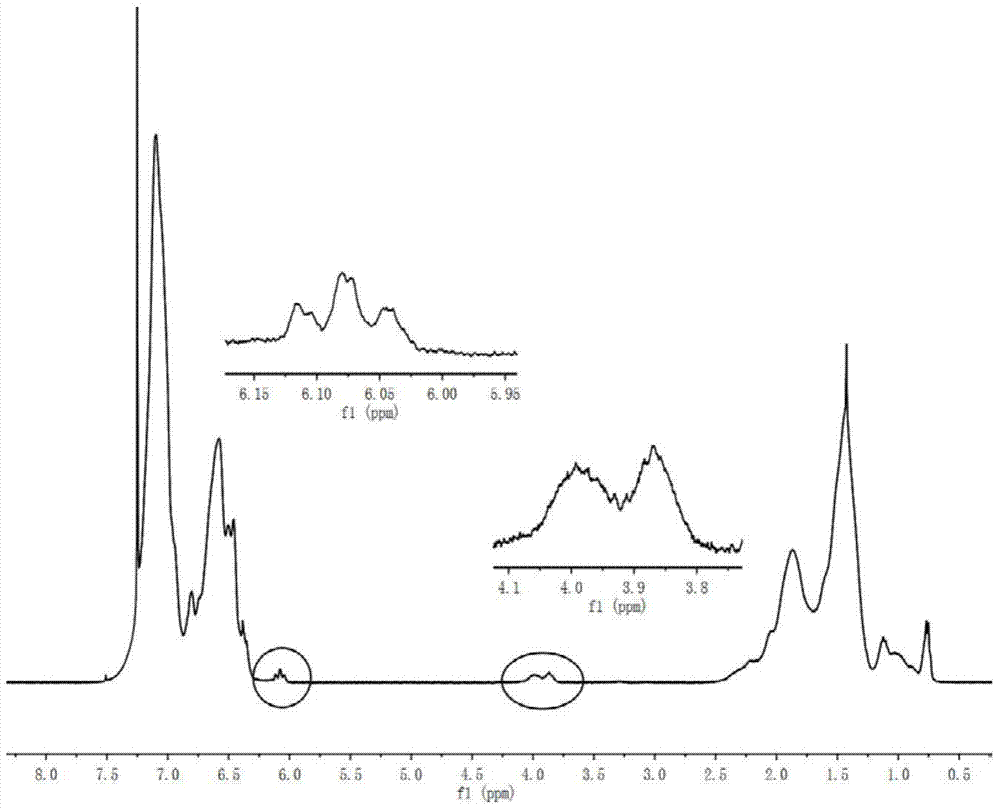
[0029] Prepare 8g of polystyrene active anionic polymer, design molecular weight 2000, polymer concentration is 10g per 100ml cyclohexane, pass through 0.4ml of ethylene oxide to react for 30min, add the tetrahydrofuran solution of maleic anhydride (containing maleic anhydride 0.771g ), reacted at 40°C for 30min, and the resulting product was precipitated with HCl ethanol solution, dried in a vacuum oven at 50°C, and then characterized. It is measured that Mn=2100, MWD=1.12, and the capping rate is 80%.
Embodiment 2
[0031] Prepare 8g of polystyrene active anionic polymer, design molecular weight 2000, polymer concentration is 10g per 100ml cyclohexane, pass through 0.3ml of ethylene oxide to react for 30min, add the tetrahydrofuran solution of maleic anhydride (containing maleic anhydride 0.771g ), reacted at 20°C for 30min, and the resulting product was precipitated with HCl ethanol solution, dried in a vacuum oven at 50°C, and then characterized. It is measured that Mn=2200, MWD=1.15, and the capping rate is 77%.
Embodiment 3
[0033] Prepare 8g of polystyrene active anionic polymer, design molecular weight 2000, polymer concentration is 10g per 100ml cyclohexane, pass through 0.4ml of ethylene oxide to react for 30min, add the tetrahydrofuran solution of maleic anhydride (containing maleic anhydride 0.771g ), reacted at 60°C for 30min, and the resulting product was precipitated with HCl ethanol solution, and then placed in a vacuum oven at 50°C for characterization. It is measured that Mn=2300, MWD=1.17, and the end-capping rate is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com