Method for preparing gamma-valerolactone by transferring and hydrogenating levulinic acid and ester thereof
A technology for levulinic acid and transfer hydrogenation, which is applied in the production of bulk chemicals and organic chemistry, can solve the problems of increasing the difficulty of separation of target products, flammability and explosion of peroxidized products, and complex reaction systems, etc., to achieve strong Potential for industrial application, easy to operate, and efficient reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
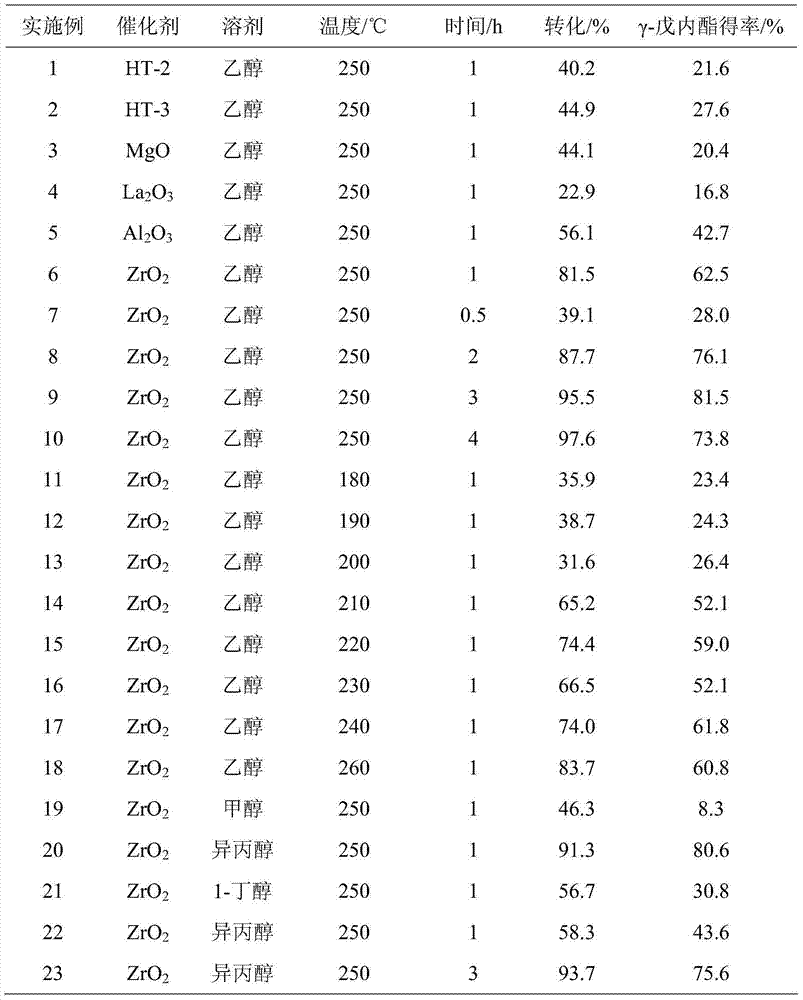
[0023] Add 2 g of ethyl levulinate and 38 g of ethanol (5 wt%) into a 100 mL autoclave, and then add 1 g of Al 2 o 3 , MgO, HT-2, HT-3, ZrO 2 , La 2 o 3 As a catalyst, seal, stir evenly (500rpm), heat to 250°C and keep for 1h, finish the reaction, cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection, detection of different catalysts The results are listed in Table 1, numbered 1-6.
Embodiment 7~10
[0025] Add 2 g of ethyl levulinate and 38 g of ethanol (5 wt%) into a 100 mL autoclave, and then add 1 g of ZrO 2 As a catalyst, seal it, stir evenly (500rpm), heat to 250°C and keep it for 0.5~4h, finish the reaction, cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection, different catalysts The test results are listed in Table 1 with serial numbers 7-10.
Embodiment 11~18
[0027] Add 2 g of ethyl levulinate and 38 g of ethanol (5 wt%) into a 100 mL autoclave, and then add 1 g of ZrO 2 As a catalyst, seal, stir evenly (500rpm), heat to 180-250°C and keep for 1h, finish the reaction and cool to room temperature and take samples, use GC-MS (Shimadzu) and GC (Agilent) for qualitative and quantitative detection, different catalysts The test results are listed in Table 1 with serial numbers 11-18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com