Method and means for sample preparation
A sample, a technique in a sample, applied in the field of depleting undesired molecules and/or enriching desired molecules, separation materials comprising magnetic particles, and depletion of high-abundance large proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
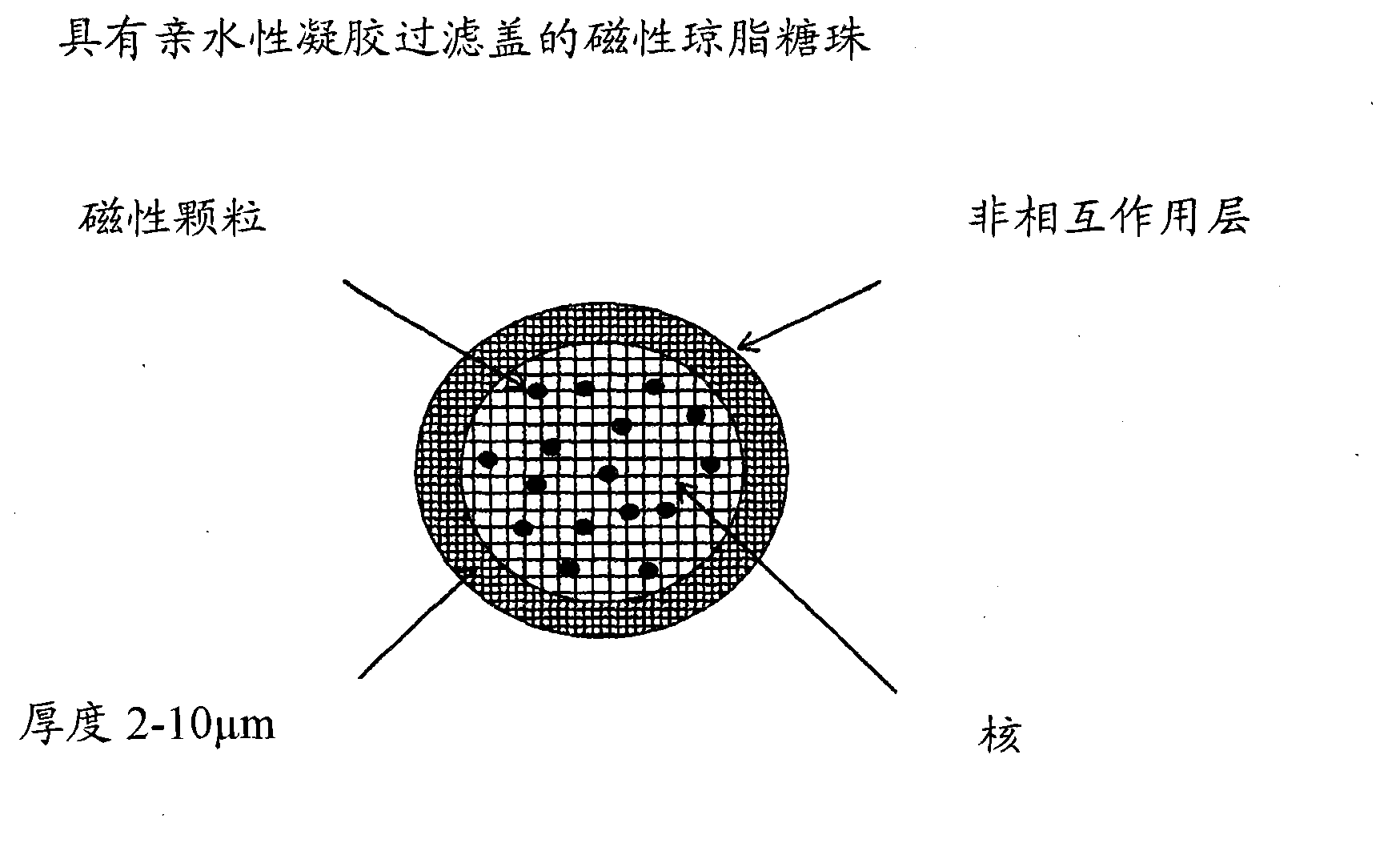
[0033] Example 1: -SO in bead-based cores 3 - Ligand preparation of agarose magnetic shell medium
[0034] This example illustrates figure 1 Synthesis of the indicated beads.
[0035] Preparation of magnetic agarose beads
[0036] Approximately 50 g of iron oxide particles (particle size 1.5 μm) were added to an agarose solution containing agarose (117 g) and water (500 g). Adjust the solution to 95 °C. This solution was then added to a solution of toluene (1420ml) and ethylcellulose (106g) in a vessel equipped with a stirrer while maintaining the temperature at 75°C. Increase mixer speed until desired particle size is achieved. The emulsion was then cooled to room temperature. Wash the beads with ethanol and water.
[0037]Add water (100 mL) to the washed beads (500 mL), Na 2 SO 4 (74g), 50% NaOH (6mL) and NaBH 4 (0.5g). The temperature was raised to 50°C, and epichlorohydrin (61 mL) and sodium hydroxide (42 mL) were added. After the addition was complete, the ...
Embodiment 2
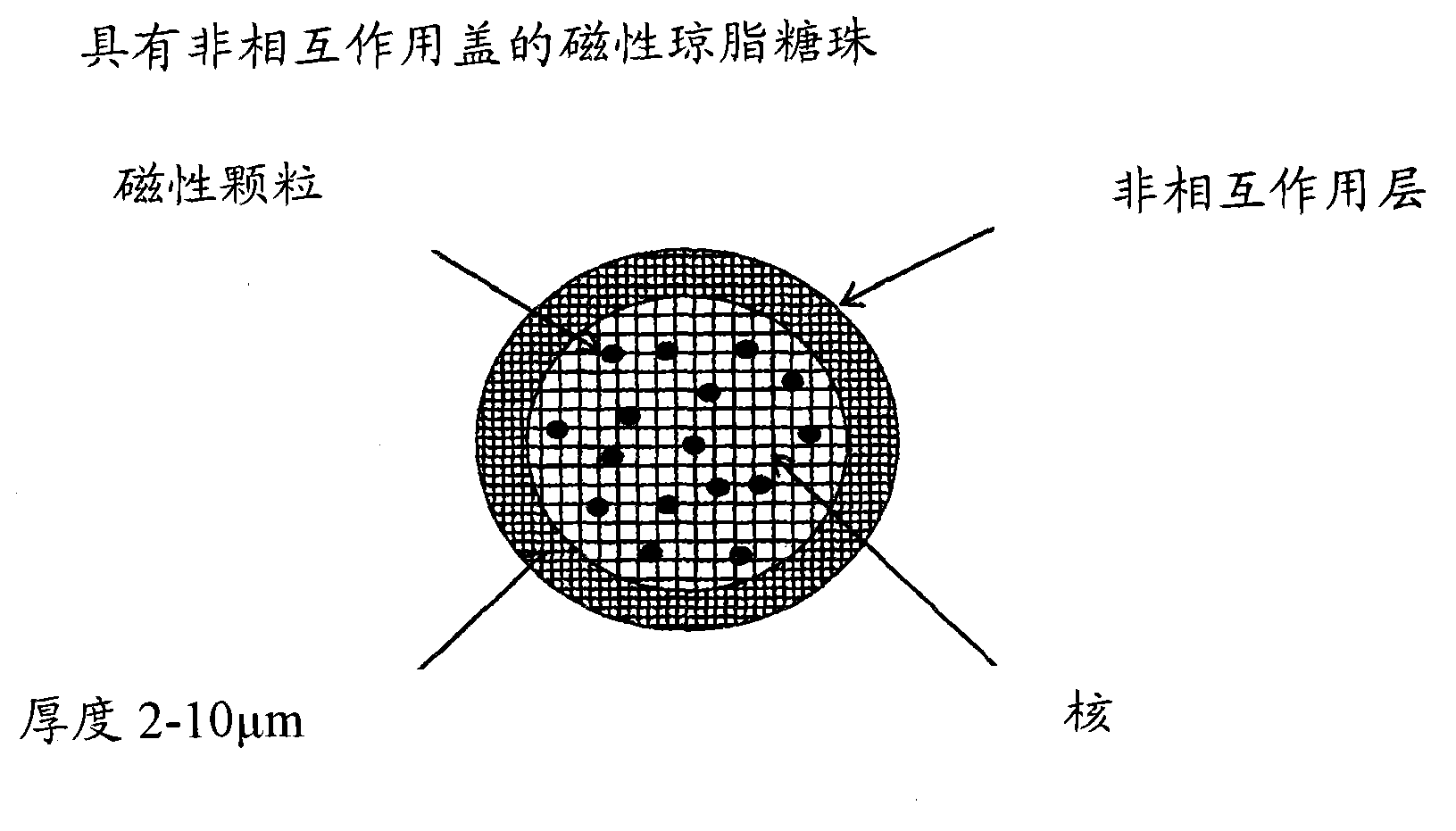
[0047] Example 2: Based on -SO in the bead core 3 - Ligand preparation of dextran magnetic shell media
[0048] This example illustrates figure 2 Synthetic procedure for the indicated beads. The synthesis was designed to obtain beads with a porosity comparable to Sephadex G-50 (separation range from 1500 to 30000 g / mol) but with iron oxide particles and ligands in the bead core.
[0049] Production of dextran magnetic beads with porosity comparable to Sephadex G-50
[0050] Add approximately 20 g of iron oxide particles (particle size 1.5 μm) to a solution containing water (200 mL), 50% NaOH (13 ml), NaBH 4 (0.5g) and dextran TF (94g) in a dextran solution. The solution was heated to 50°C and then added to a solution of dichloroethane (200ml) and cellulose acetate butyrate (12g) in a vessel equipped with a paddle stirrer. Increase mixer speed until desired particle size is achieved. Epichlorohydrin (13 mL) was then added and the reaction was continued at 50°C for 16 ...
Embodiment 3
[0053] Example 3: Chromatographic evaluation of the samples described in Example 1
[0054] To confirm that proteins with a molecular weight greater than about 10000 g / mol are excluded from the magnetic beads produced according to Example 1, the breakthrough capacity of a number of proteins was tested. A sample based on magnetic agarose beads (see Example 1) is packed into a suitable column through which the protein solution is pumped. Adjust mobile phase conditions to acidic pH to allow all proteins to adsorb to the nuclear ligand (-SO 3 - ).
[0055] The magnetic agarose shell medium (sample produced in Example 1) to be studied in terms of breakthrough capacity was filled into a HR5 / 2 column, and after equilibrating with buffer, the sample solution was pumped through the column at a flow rate of 0.2 mL / min. The breakthrough capacity was evaluated at 10% of the highest UV detector signal (280 nm). The maximum UV signal was estimated by pumping the test solution directly i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com