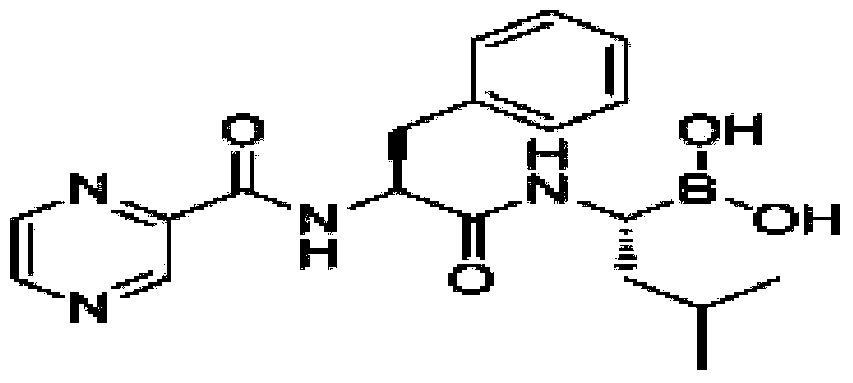
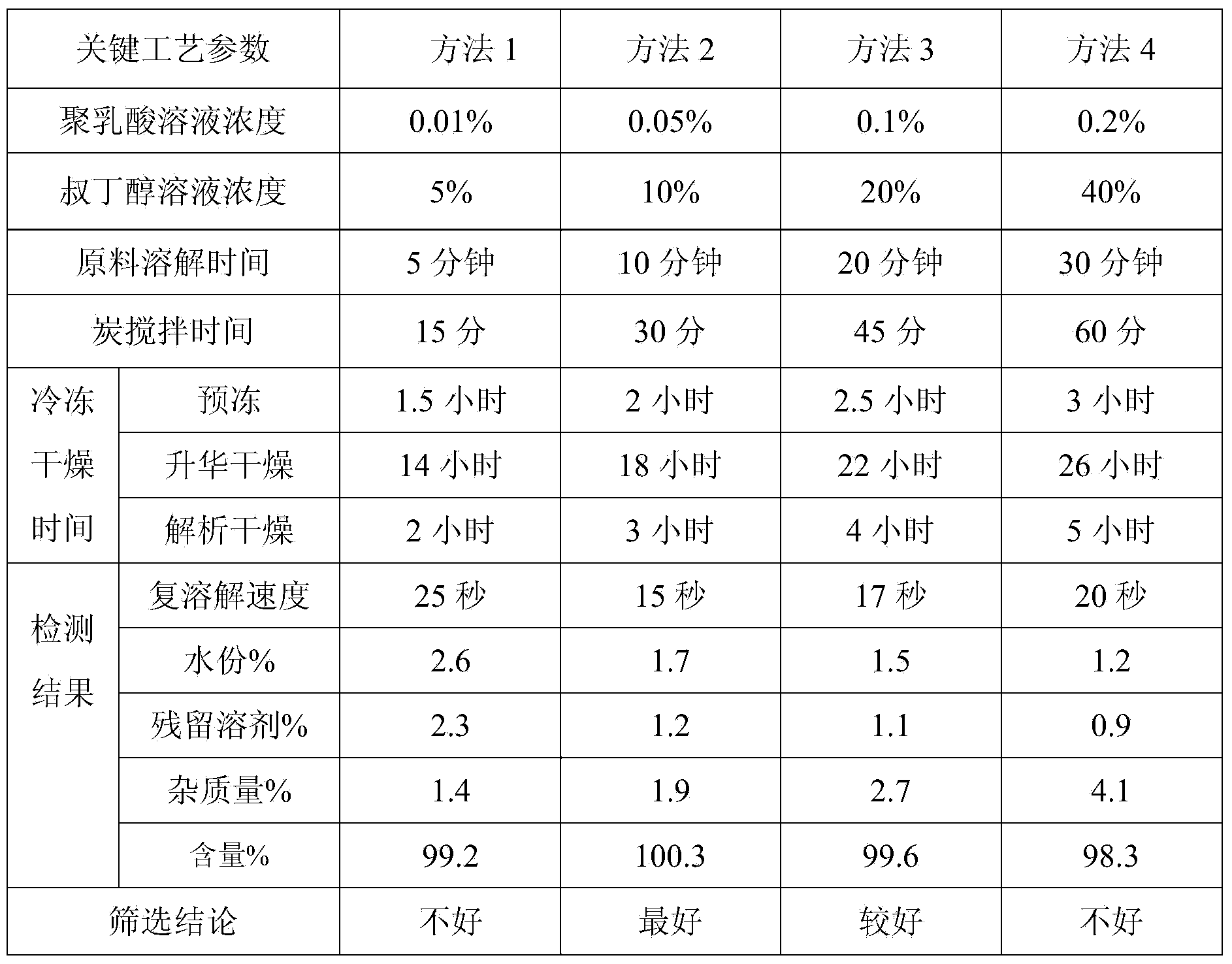
Preparation method for bortezomib for injection
A technology for bortezomib and injection, which is applied in the field of pharmaceutical preparations, and can solve the problems of no increase in reconstitution speed of bortezomib freeze-dried powder injection, insoluble bortezomib, and increased impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] The pH value of the solution is 4.0-7.0.
[0041] Its preparation method comprises the following steps:
[0042] 1) Weigh polylactic acid and tert-butanol, add a certain amount of water for injection to dissolve, and obtain a mixed solution of polylactic acid and tert-butanol;
[0043] 2) Weigh the bortezomib raw material, add it to the above solution, and stir until completely dissolved;
[0044] 3) Weigh mannitol, add it to the solution in which the above raw material drug has been completely dissolved, add water for injection to 70% of the total amount, and stir rapidly until it is completely dissolved;
[0045] 4) Add 5g of activated carbon and stir for 20 minutes, then filter out the activated carbon;
[0046] 5) Add water for injection to 9.5L, adjust the pH value to 4.0-7.0 with sodium hydroxide or hydrochloric acid, and add water for injection to 10L;
[0047] 6) After filling, use vacuum freeze-drying technology to freeze-dry. The freeze-drying p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com