Reactive dye
A reactive dye, CH2 technology, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems of high concentration of suspended solids, high chroma of dyeing and finishing wastewater, high biochemical oxygen demand, etc., and achieve good dyeing depth , High dye utilization rate, high coloring rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
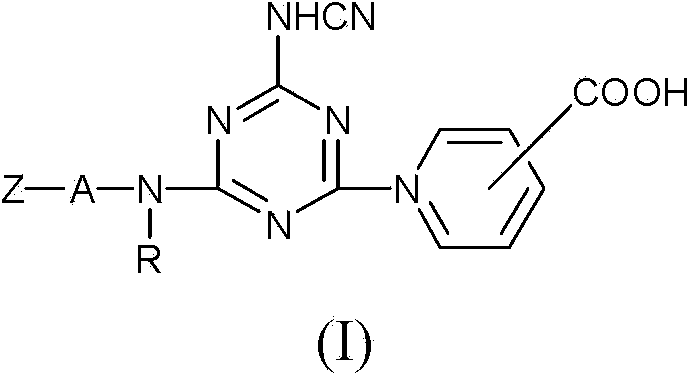
[0043] Take 18.4 parts of cyanuric chloride and disperse in 150 parts of water at 0°C, then add 4.2 parts of cyanamide powder, and use sodium hydroxide (NaOH) solution to control the pH value of the reaction solution between 10 and 11 , after 60 minutes, add 52.3 parts of 3-ureido-4-[2-sulfo-4-(β-ethylsulfonyl sulfate)-phenylazo]-aniline (3-ureido-4-[2 -sulfo-4-(β-sulphatoethyl sulphonyl)-phenylazo]-aniline), controlled at 40~55°C, pH 6~8, after reacting for about 4 hours, add 12.4 parts of nicotinic acid (Nicotinic acid), control at Carry out reaction at 60~65 ℃ for 5 hours, obtain yellow product through salting-out, extraction, filtration and drying, and by the usual identification technique in this field, to confirm that the product of the structure shown in following formula (1) is obtained: λmax=423nm, The molecular weight measured by liquid chromatography / mass spectrometer (LC / MS) is 764.lg / mole.
[0044]
Embodiment 2
[0046] Take 18.4 parts of cyanuric chloride and disperse in 150 parts of water at 0°C, then add 4.2 parts of cyanamide powder, and use sodium hydroxide (NaOH) solution to control the pH value of the reaction solution between 10 and 11 , After keeping for 60 minutes, add 52.3 parts of 3-acetamide-4-[2-sulfo-4-(β-sulfuric acid ethylsulfonyl)-phenylazo]-aniline (3-acetylamino-4-[2 -sulfo-4-(β-sulphatoethylsulphonyl)-phenylazo]-aniline), controlled at 40~55°C, pH 6~8, after reacting for about 4 hours, add 31.0 parts of niacin (Nicotinic acid), add sodium acetate Control the pH value of the reaction solution between 5 and 6, react at 85 to 90°C for 3 hours, obtain a yellow product through salting out, extraction, filtration and drying, and use the usual identification techniques in this field to confirm that the following formula ( 2) The product with the structure shown: λmax=419nm, the molecular weight measured by liquid chromatography / mass spectrometer (LC / MS) is 789.3g / mole.
...
Embodiment 3
[0049] Take 57.3 parts of 3-ureido-4-[1-sulfo-6-(β-ethylsulfone sulfate)-naphthyl-2-azo]-aniline (3-ureido-4-[1-sulfo-6 -(β-sulfatoethylsulfone)-naphthalenyl-2-azo]-aniline), instead of the 3-ureido-4-[2-sulfo-4-(β-sulfatoethylsulfonyl)-phenylene of Example 1 Nitrogen]-aniline (3-ureido-4-[2-sulfo-4-(β-sulphatoethylsulphonyl)-phenylazo]-aniline), the yellow product was obtained according to the same preparation procedure, and was confirmed by the usual identification techniques in the art The product with the structure shown in the following formula (3): λmax=425nm, the molecular weight measured by liquid chromatography / mass spectrometer (LC / MS) is 814.0g / mole.
[0050]
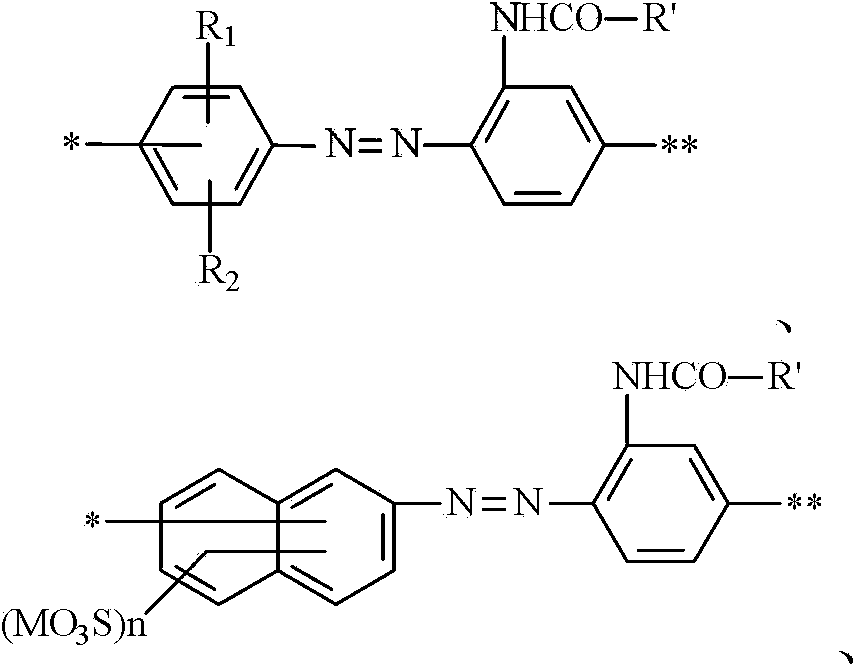
[0051] Embodiment 4-6 Referring to the synthesis method of Examples 1-3, the yellow dye structural formula of Examples 4-6 can be obtained as follows:
[0052]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap