A kind of plating flux for steel wire hot-dip galvanized aluminum-magnesium alloy
A hot-dip galvanizing and aluminum-magnesium alloy technology, applied in hot-dip galvanizing process, metal material coating process, coating, etc., can solve the difference in the specific surface reaction rate requirements of the fluxing agent, and the fluxing agent cannot meet the requirements of the fluxing The requirements of the coating agent, the poor bonding strength between the coating and the steel wire, etc., to achieve excellent corrosion resistance, low cost, and avoid the effect of coating falling off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
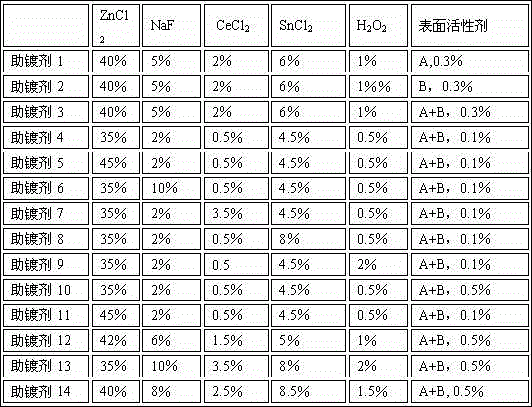
[0033] Hot-dip galvanizing-5% aluminum-1% magnesium alloy flux formulation, the components are as follows:
[0034] 45% ZnCl 2 , 2% NaF, 0.5% CeCl 2 , 4.5% SnCl 2 , 0.5%H 2 o 2 , 0.1% surfactant (fatty alcohol ether sodium sulfate and sodium dodecylsulfonate mixed according to the weight ratio of 1:1), the balance is tap water.
[0035]After degreasing, pickling and alkali cleaning, the steel wire is immersed in the plating flux described at 80°C at a speed of 7.5m / min, dried at 120°C, and then hot dipped in a zinc-aluminum-magnesium alloy solution for 1min. After cooling, the surface of the steel wire has no missing coating, uniform coating and good finish. The thickness of the coating is about 60-90μm. The results of the neutral salt spray test show that the time for rust spots to appear is 430 hours, and the time for rust spots to appear in traditional hot-dip galvanizing is 140 hours. The service life is much higher than that of hot-dip galvanized pure zinc.
Embodiment 2
[0037] Hot-dip galvanizing-5% aluminum-2% magnesium alloy flux formulation, the components are as follows:
[0038] 42% ZnCl 2 , 6% NaF, 1.5% CeCl 2 , 5% SnCl 2 , 1%H 2 o 2 , 0.3% surfactant (sodium fatty alcohol ether sulfate and sodium lauryl sulfate), the balance being tap water.
[0039] After degreasing, pickling, and alkali cleaning, the steel wire is immersed in the plating flux described at 75°C at a speed of 9 m / min, dried at 100°C, and then hot-dipped in a zinc-aluminum-magnesium alloy solution for 1 min. After cooling, the surface of the steel wire has no missing coating, uniform coating and good finish. The thickness of the coating is about 70-100μm. The results of the neutral salt spray test show that the time for rust spots to appear is 570 hours, and the time for rust spots to appear in traditional hot-dip galvanizing is 140 hours. The service life is much higher than that of hot-dip galvanized pure zinc.
Embodiment 3
[0041] Hot-dip galvanizing-6% aluminum-3% magnesium alloy flux formulation, the components are as follows:
[0042] 35% ZnCl 2 , 10% NaF, 3.5% CeCl 2 , 8% SnCl 2 , 2%H 2 o 2 , 0.5% surfactants (sodium fatty alcohol ether sulfate and sodium lauryl sulfate), the balance being tap water.
[0043] After degreasing, pickling and alkali cleaning, the steel wire is immersed in the plating flux described at 75°C at a speed of 10 m / min, dried at 120°C, and then hot-dipped in a zinc-aluminum-magnesium alloy solution for 3 minutes. After cooling, the surface of the steel wire has no missing coating, uniform coating and good finish. The thickness of the coating is about 80-100μm. The results of the neutral salt spray test show that the time for rust spots to appear is 790 hours, and the time for rust spots to appear in traditional hot-dip galvanizing is 140 hours. The service life is much higher than that of hot-dip galvanized pure zinc.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com