Device and method for shortening ketalation time in ibuprofen synthesis process
A technology of reaction time and synthesis process, applied in organic chemistry, chemical industry, sustainable manufacturing/processing, etc., can solve problems such as restricting ibuprofen production cycle, limiting ibuprofen production capacity, long reaction time, etc., and achieves easy washing Effects of operation, increasing reaction contact area and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
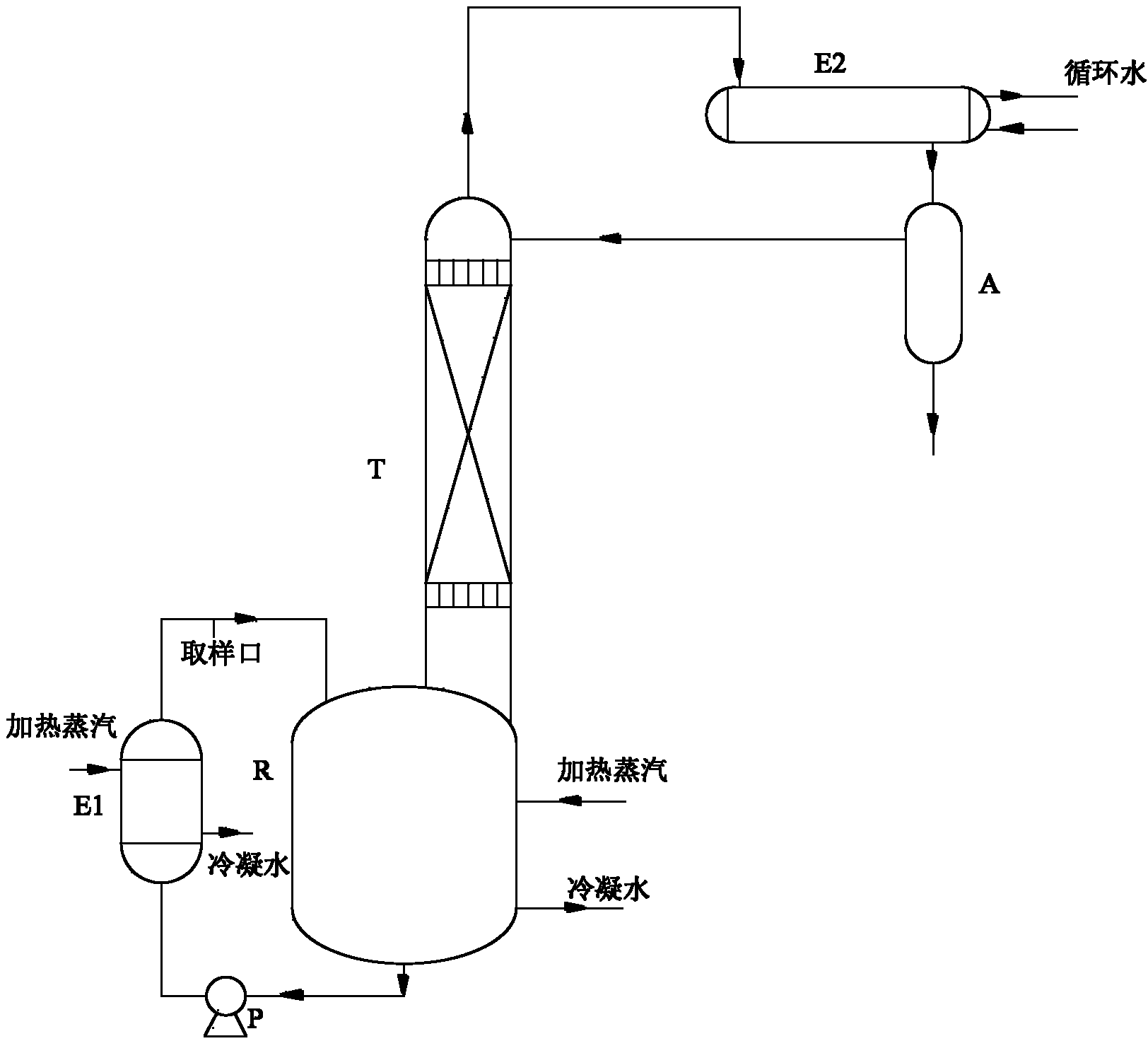
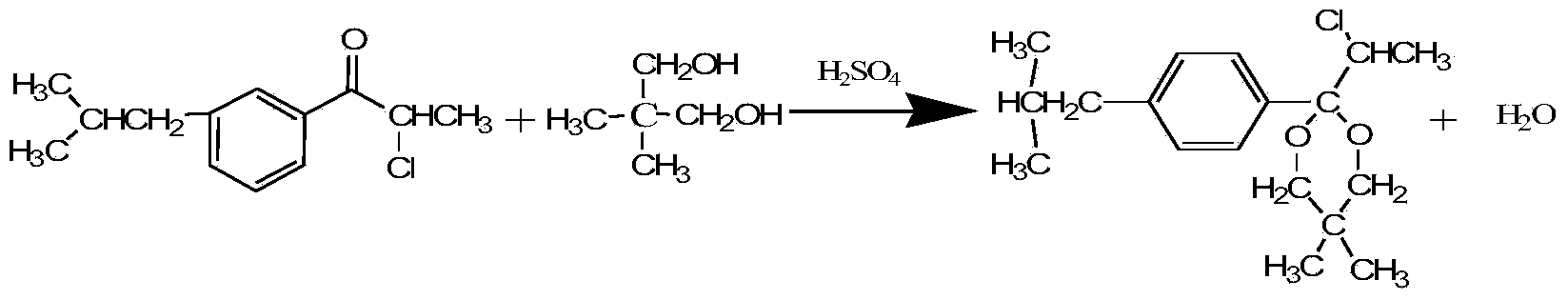
[0039] Feeding: 200g of petroleum ether, 105.3g of ketone, 71.3g of neopentyl glycol, and 9.3mL of 30% dilute sulfuric acid. After being heated by the steam in the jacket of the reaction kettle and the reboiler (E1), the temperature of the liquid in the reaction kettle (R) rises from 50°C to 85°C, the temperature at the bottom of the tower is 70°C, and the mass content of ketone in the bottom of the tower is 1.6%. The temperature at the top of the tower was 62°C, the mass content of neopentyl glycol in the bottom water layer of the reflux tank (A) was 0.8%, and the ketalization reaction time was 8 hours.
Embodiment 2
[0041] Feeding: 300g of petroleum ether, 158g of ketone, 107g of neopentyl glycol, 14mL of 30% dilute sulfuric acid. After being heated by the steam in the jacket of the reactor (R) and the reboiler (E1), the temperature of the liquid in the reactor (R) rises from 50°C to 92°C, the temperature at the bottom of the tower is 79°C, and the mass content of ketone in the bottom of the tower is 1.4%, the temperature at the top of the tower is 69°C, the mass content of neopentyl glycol in the water layer at the bottom of the reflux tank (A) is 0.5%, and the ketalization reaction time is 7.8 hours.
Embodiment 3
[0043] Feed: 3kg of petroleum ether, 1.6kg of ketone, 1.1kg of neopentyl glycol, 145mL of 30% dilute sulfuric acid. After being heated by the steam in the jacket of the reactor (R) and the reboiler (E1), the temperature of the liquid in the reactor (R) rises from 50°C to 97°C, the temperature at the bottom of the tower is 88°C, and the mass content of ketone in the bottom of the tower is 1.3%, the temperature at the top of the tower is 80°C, the mass content of neopentyl glycol in the water layer at the bottom of the reflux tank (A) is 0.83%, and the ketalization reaction time is 7.2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com