Diene-butyl ferrocene and preparation method thereof
A dienyl butyl ferrocene and allyl Grignard technology, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve problems such as hindering throughput production and unstable raw materials required for synthesis, and achieve Novel structure, mild reaction conditions, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] raw material preparation
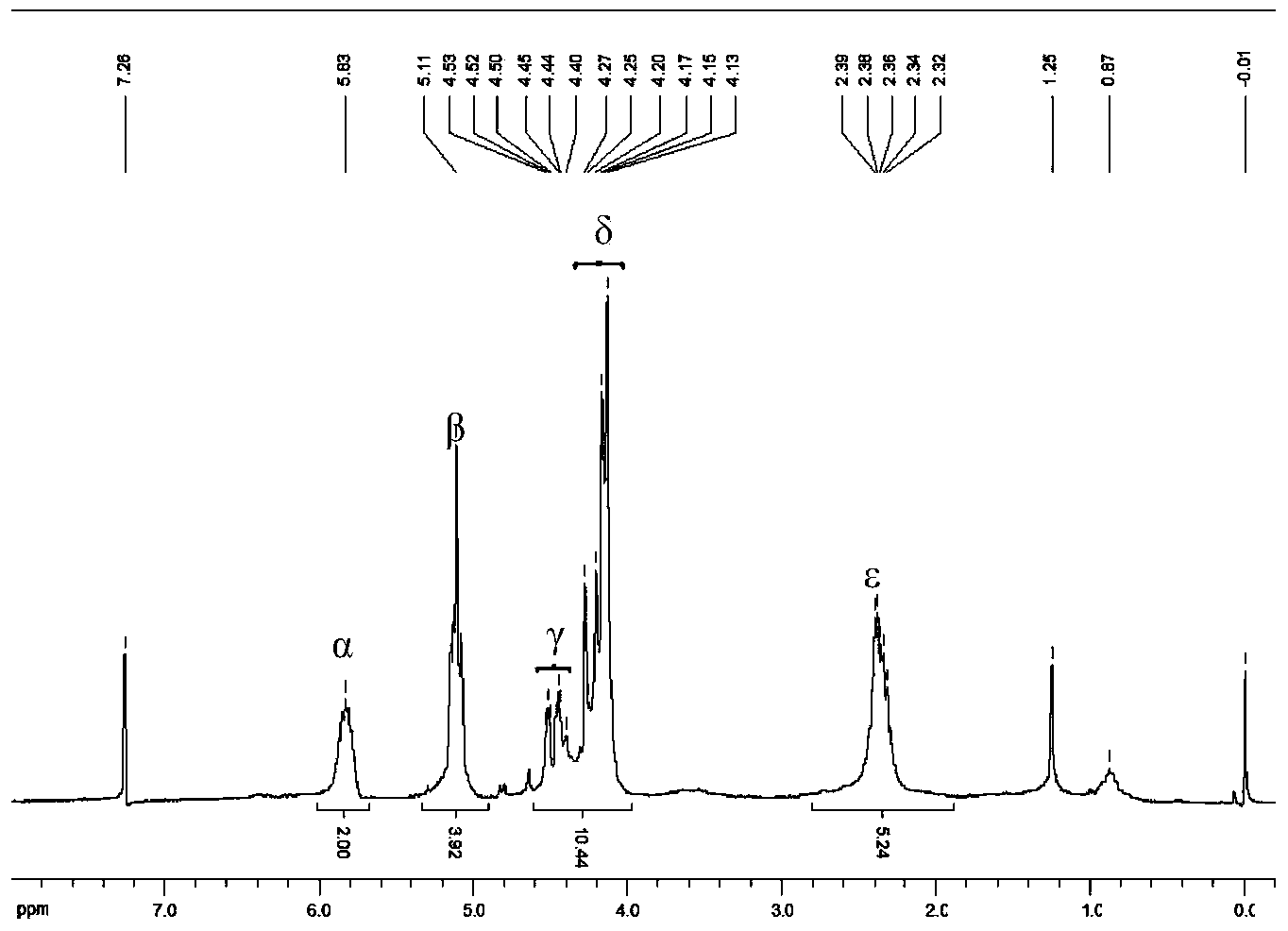
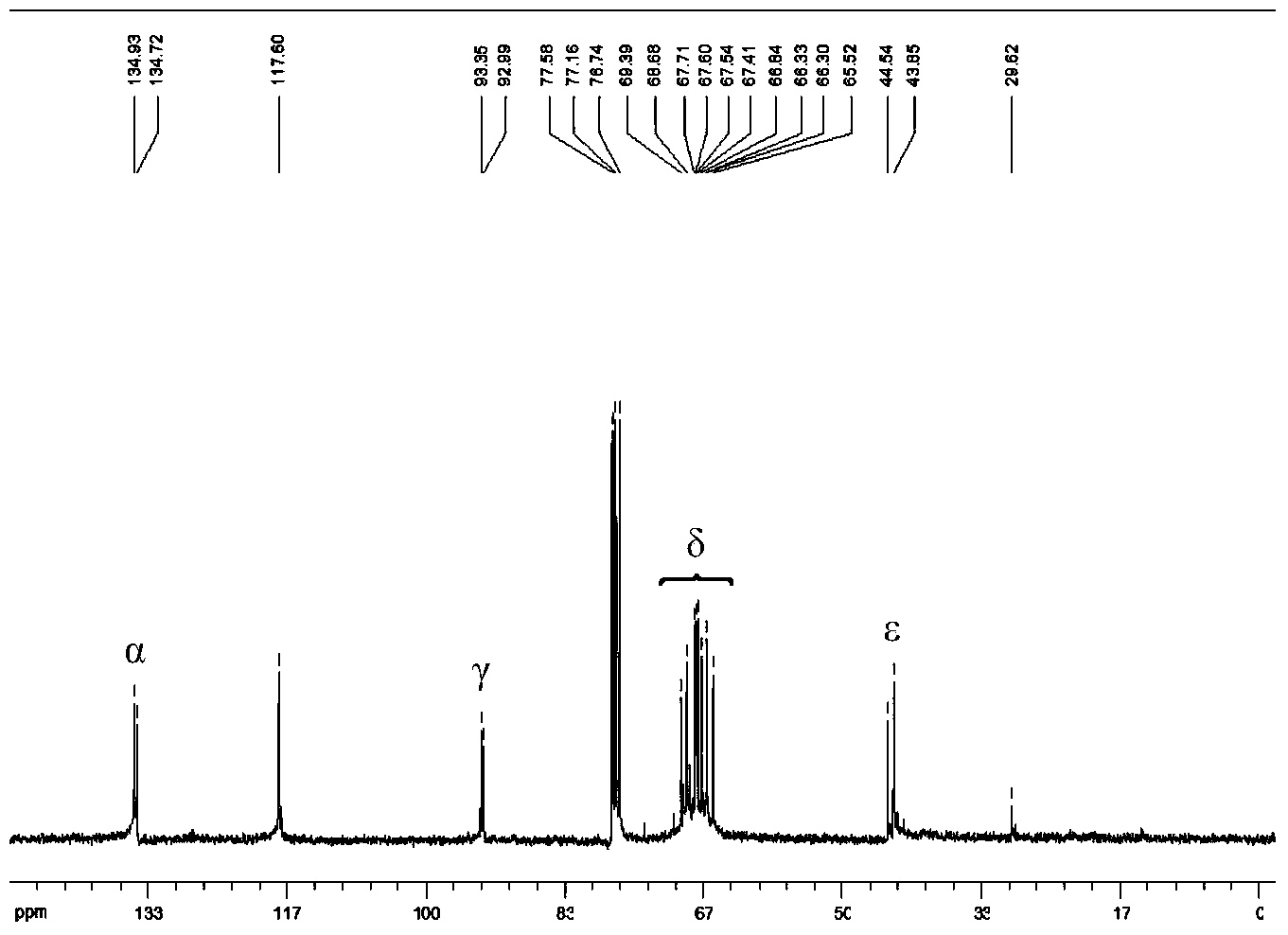
[0036] Add 3.100g (16.6mmol) vacuum-dried ferrocene and 12.0mL n-hexane to a dry Schlenk bottle, and stir to form a suspension; then add 25.0mL n-butyl lithium (1.6M, 40.0mmol), and then Add 6.0mL tetramethylethylenediamine (40.0mmol) dropwise within 60min, and continue to stir and react at room temperature for 16h; filter the reaction mixture, and wash the orange-red powder three times with 15.0mL n-hexane to obtain 1 , 1'-dilithium ferrocene is about 15.0mmol, add 35.0mL n-hexane to it, and stir to form a suspension; add 2.8mL (36.0mmol) dimethylformamide to 12mL anhydrous ether, and place in an ice-water bath Add this solution dropwise to the above suspension under cooling (about 30min); remove the ice-water bath after the dropwise addition, let it rise to room temperature naturally and stir for about 30min, add 50.0mL (4.0M, 0.2mol) hydrochloric acid, and continue the reaction for 15min After standing still, the layers were separated, and...
Embodiment 2
[0048] raw material preparation
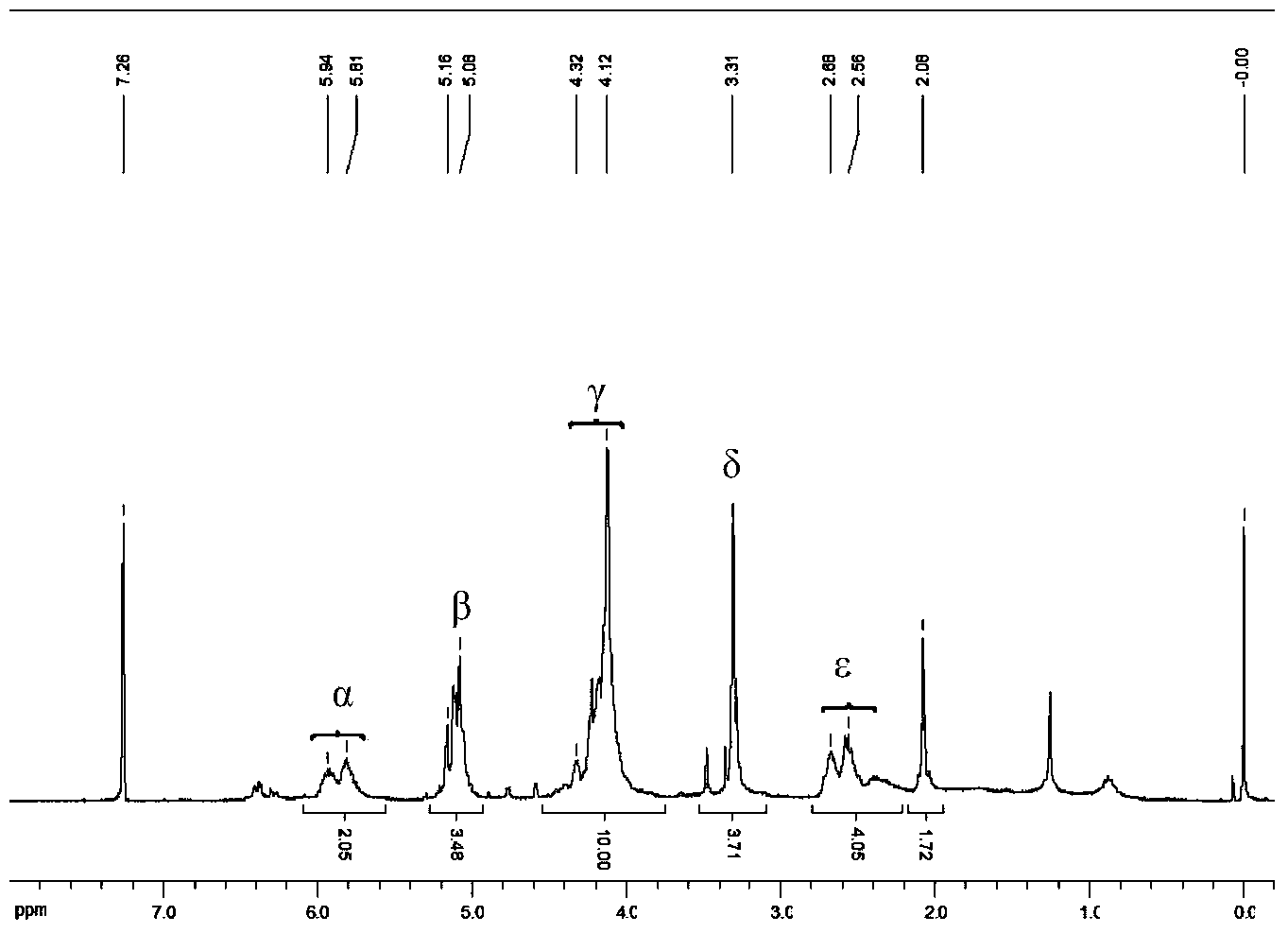
[0049] Add 3.071g (16.5mmol) vacuum-dried ferrocene and 12.0mL n-hexane to a dry Schlenk bottle, stir to form a suspension; then add 25.0mL n-butyl lithium (1.6M, 40.0mmol), and then Add 6.0 mL of tetramethylethylenediamine (40.0 mmol) dropwise within 60 minutes, and continue to stir and react at room temperature for 16 hours; filter the reaction mixture, and wash the obtained orange-red powder three times with 15.0 mL of n-hexane to obtain 1, 1'-Lithium ferrocene is about 15.0mmol, add 35.0mL n-hexane to it, stir to form a suspension; add 2.8mL (36.0mmol) dimethylformamide to 12mL anhydrous ether, cool in an ice-water bath Add this solution dropwise to the above suspension (about 30min); after the dropwise addition, remove the ice-water bath, naturally warm up to room temperature and stir for about 30min, add 50.0mL (4.0M, 0.2mol) hydrochloric acid to continue the reaction for 15min, and then separate The aqueous layer was extracted three ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com