System and technology for synthesis of methanol and co-production of methane by using synthesis gas
A technology for synthesizing methanol and methane, which is applied in the chemical industry, can solve problems such as environmental pollution and resource waste, achieve good peak-shaving operations, eliminate carbon emissions, and achieve the effects of diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
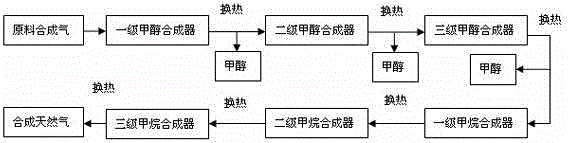
[0033] The process flow of synthesizing methanol from synthesis gas and co-producing methane figure 1 shown. In this embodiment, according to the composition of raw material gas, the synthesis gas is firstly passed through a three-stage methanol synthesis reactor, and then through a three-stage methanation reactor. The methanol reactor and the methanation reactor are all connected in series.
[0034] The composition of raw syngas is: CO 28.0%; CO 2 2.18%; H 2 64.25%; N 2 0.1%; CH 4 5.47%, the amount of feed gas is 100L / h, the reaction temperature of synthetic methanol is 220°C, the pressure is 5.0MPa, and the reaction space velocity is 5000h -1 , the synthesis gas enters the methanation reactor after passing through the three-stage synthesis methanol reactor. The specific composition of the reaction tail gas at the outlet of the third-stage methanol synthesis reactor after condensation and gas-liquid separation is: CO4.90%; CO 2 8.20%; H 2 46.74%; N 2 0.72%; CH ...
Embodiment 2
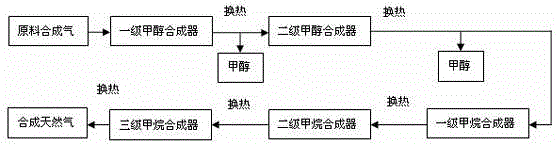
[0036] The process flow of synthesizing methanol from synthesis gas and co-producing methane figure 2 As shown, in this example, according to the composition of raw material gas, the synthetic gas is firstly passed through a two-stage methanol synthesis reactor, and then through a three-stage methanation reactor. The methanol reactor and the methanation reactor are all connected in series.
[0037] The composition of raw syngas is: CO 19.08%; CO 2 1.30%; H 2 64.41%; N 2 0.29%; CH 4 14.62%, the amount of feed gas is 100L / h, the ratio of hydrogen to carbon is 3.10, the reaction temperature of synthetic methanol is 220℃, the pressure is 5.0MPa, and the reaction space velocity of primary methanol synthesis is 10000h -1 , the synthesis gas enters the methanation reactor after passing through the secondary methanol synthesis reactor. The specific composition of the reaction tail gas at the outlet of the second-stage methanol synthesis reactor after condensation and gas-liqui...
Embodiment 3
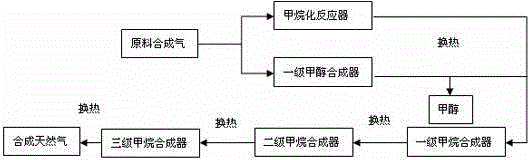
[0039] The process flow of synthesizing methanol from synthesis gas and co-producing methane image 3 shown. In this embodiment, according to the composition of the raw material gas, the synthetic gas is divided into two routes and enters a synthesis methanol reactor and a primary methanation reactor respectively, and then passes through a three-stage methanation reactor.
[0040] The amount of raw gas is 100L / h, and the composition of raw syngas is: CO 16.65%; CO 2 1.504%; H 2 72.888%; N 2 0.304%; CH 4 8.66%, 20L / h raw synthesis gas enters the methanol synthesis reactor, and another 80L / h raw synthesis gas enters the methanation reactor after heat exchange. The reaction temperature for synthesizing methanol is 220°C, the pressure is 5.0MPa, and the reaction space velocity is 7000h -1 , the specific composition of the reaction tail gas at the outlet of the synthetic methanol reactor after condensation and gas-liquid separation is: CO 9.72%; CO 2 1.64%; H 2 75.56%;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com