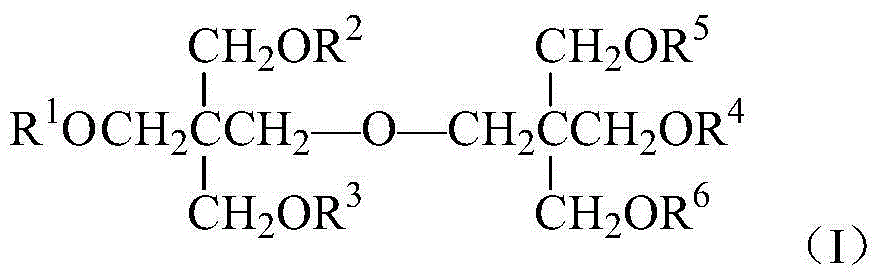
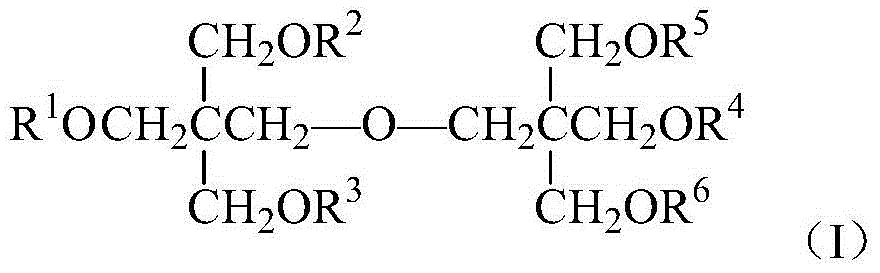
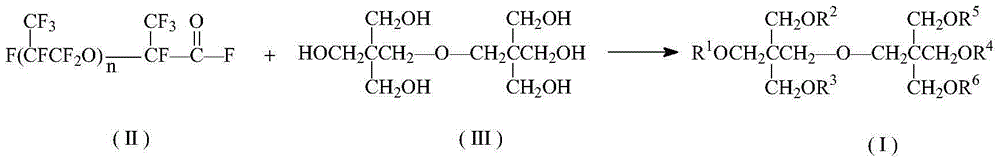Oxa perfluorocarboxylic acid dipentaerythritol ester and preparation and application thereof
A technology of dipentaerythritol ester and dipentaerythritol, which is applied in the field of dipentaerythritol oxaperfluorocarboxylate and its preparation and application, and achieves the effects of simple preparation method, high surface activity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser (an anhydrous CaCl 2 Drying tube) In a dry 250mL four-necked flask, add 5.08g (0.02mol) of dipentaerythritol, 25mL of DMF, and 4.1g (0.04mol) of triethylamine. Stir, heat to 55°C, add 15.54g (0.04mol) hexafluoropropylene oxide oligomer mixture (HFPO) dropwise while stirring n . After the dropwise addition was completed, the temperature was continued to rise to 80°C, and the reaction was stirred for 8h. The resulting product was first washed twice with 25mL5%HCl solution, and then washed with 50mL5%NaHCO 3 The solution was washed once, extracted with 25mL ether after washing with water, and the upper organic layer was obtained by liquid separation, and the solvent was distilled off under reduced pressure to obtain a light yellow solid with a yield of 76.1% (based on hexafluoropropylene oxide oligomer mixture) .
[0031] FTIR analysis: 3300cm -1 ~3500cm -1 (residual O-H stretching vibra...
Embodiment 2
[0034] The experimental method is the same as in Example 1, however, the 15.54 g hexafluoropropylene oxide oligomer mixture is changed to 13.28 g (0.04 mol) hexafluoropropylene oxide dimer. The yield was 72.0% (based on hexafluoropropylene oxide dimer).
Embodiment 3
[0036] The experimental method is the same as in Example 1, but the 15.54 g hexafluoropropylene oxide oligomer mixture is changed to 19.92 g (0.04 mol) hexafluoropropylene oxide trimer. The yield was 77.8% (based on hexafluoropropylene oxide trimer).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com