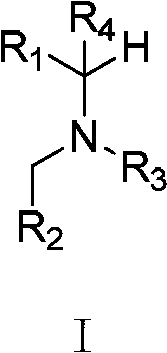
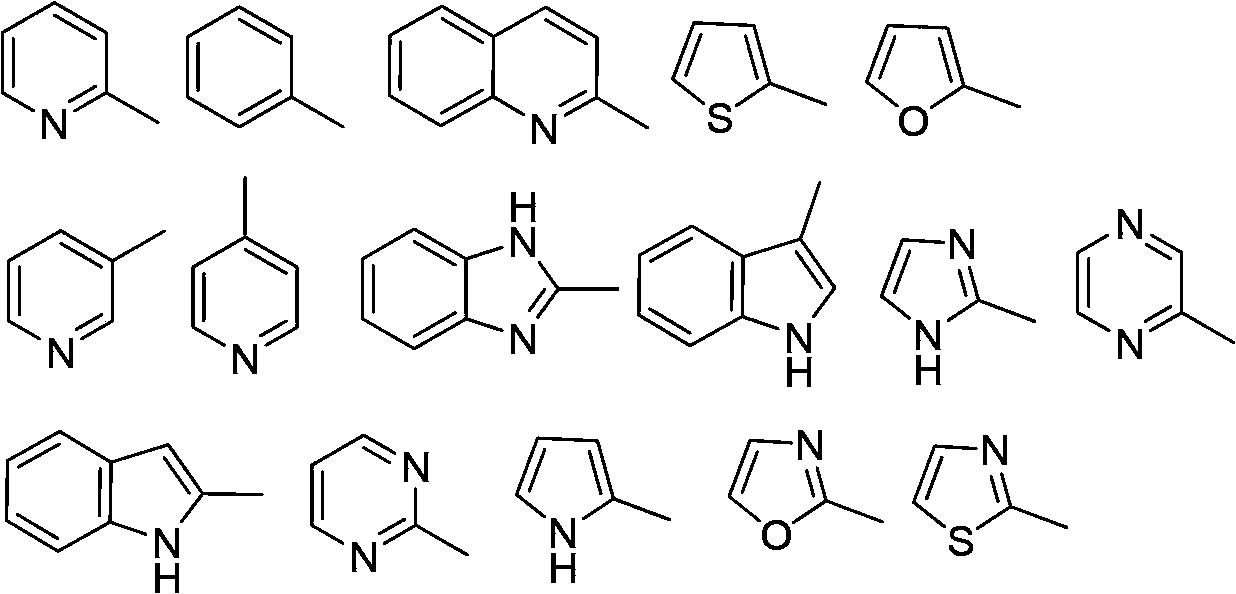
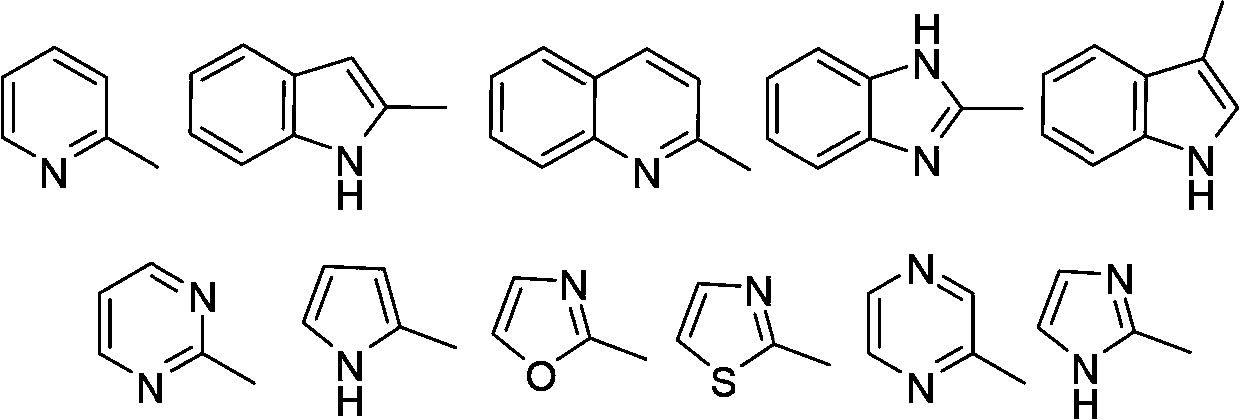
Multi-substituted amine compounds and their preparation methods and uses
A technology of polysubstituted amines and compounds, applied in the field of medicine, can solve the problem of undetectable integrase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Compound 1: tert-butyl 2-((((3-(2-cyanoethyl)pyridin-2-yl)methyl)(methyl)amino)methyl)-1H-benzimidazole-1-carboxylate ester
[0189]
[0190] Step a: 3-(2-Methylpyridin-3-yl)acrylonitrile
[0191] 3-Bromo-2-methylpyridine (1.72g, 10mmol), acrylonitrile (3.29mL, 50mmol), palladium acetate (244mg, 1mmol), tetra-n-butylammonium chloride (2.78g, 10mmol) and bicarbonate Sodium (4.2 g, 50 mmol) was dissolved in 15 mL of N,N-dimethylformamide (DMF). mixture in N 2Under the protection, it was placed in the microwave at 110°C for 5 hours. After cooling, the DMF was spin-dried, the residue was separated with water and dichloromethane (DCM), the organic phase was dried over anhydrous sodium sulfate, concentrated by filtration, and the residue was column chromatographed to obtain the title compound as a white gum (1.33 g, recovered rate of 93%).
[0192] 1 H NMR (300MHz, CDCl 3 ,ppm):δ8.52-8.50(m,1.5H),8.18(d,0.5H,J=7.8Hz),7.72(d,1H,J=7.8Hz),7.63(d,1H,J=16.5 Hz),7.37(d,0...
Embodiment 2
[0217] Compound 2: 3-(2-((((1H-benzimidazol-2-yl)methyl)(methyl)amino)methyl)pyridin-3-yl)propionitrile
[0218]
[0219] Under stirring at 0°C, 3-(2-cyanoethyl)pyridin-2-yl)methyl)(methyl)amino)methyl)-1H-benzimidazole-1-carboxylic acid tert-butyl ester ( 42 mg, 0.104 mmol) in 5 mL of dichloromethane was added trifluoroacetic acid (119 μL, 1.55 mmol), and the mixture was stirred at room temperature for 2 h. The solvent and residual trifluoroacetic acid were spin-dried, and the residue was neutralized with saturated sodium carbonate solution. After the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated, and the residue was obtained by column chromatography. The compound was light yellow gum (28 mg, yield 90%).
[0220] 1 H NMR (300MHz, CDCl 3 )δ10.15(brs,1H),8.57(d,1H,J=4.5Hz),7.65-7.62(m,3H),7.32-7.23(m,3H),3.88(s,2H),3.80(s ,2H),3.06(t,2H,J=6.9Hz),2.77(t,2H,J=6....
Embodiment 3
[0222] Compound 3: 3-(2-(((isoquinolin-1-ylmethyl)(methyl)amino)methyl)pyridin-3-yl)propionitrile
[0223]
[0224]Under stirring, 1,2 - Acetic acid (10.6 μL, 0.183 mmol) and sodium triacetoxyborohydride (116 mg, 0.549 mmol) were successively added to 2 mL of dichloroethane solution, and the mixture was stirred at room temperature for 24 h. The solvent was evaporated to dryness, and the residue was subjected to column chromatography to obtain the title compound as a yellow oil (36 mg, yield 63%).
[0225] 1 H NMR (300MHz, CDCl 3 )δ8.94-8.91(m,2H),8.52(d,1H,J=8.1Hz),8.28(d,1H,J=7.8Hz),8.15-7.95(m,4H),7.67-7.63(m ,1H),4.72(s,2H),4.36(s,2H),3.19-3.17(m,2H),2.82-2.80(m,5H); 13 C NMR (100MHz, CDCl 3 , ppm): δ157.2, 156.0, 147.4, 141.4, 137.5, 136.2, 133.4, 130.1, 127.2, 125.4, 122.9, 120.7, 118.9, 62.1, 61.8, 43.1, 27.2, 18.0; EI-MS: 316 (M + );HRMS(EI): Calculated value: C 20 h 20 N 4 (M) + :316.1688; Measured value: 316.1685.
[0226] Wherein the preparation process...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com