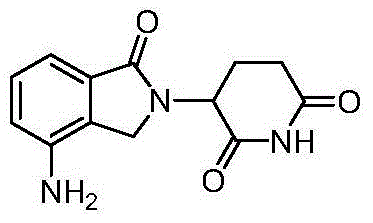
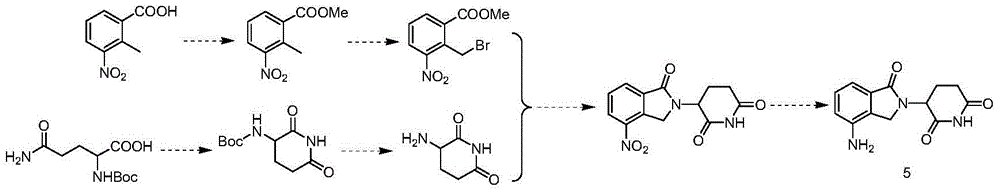
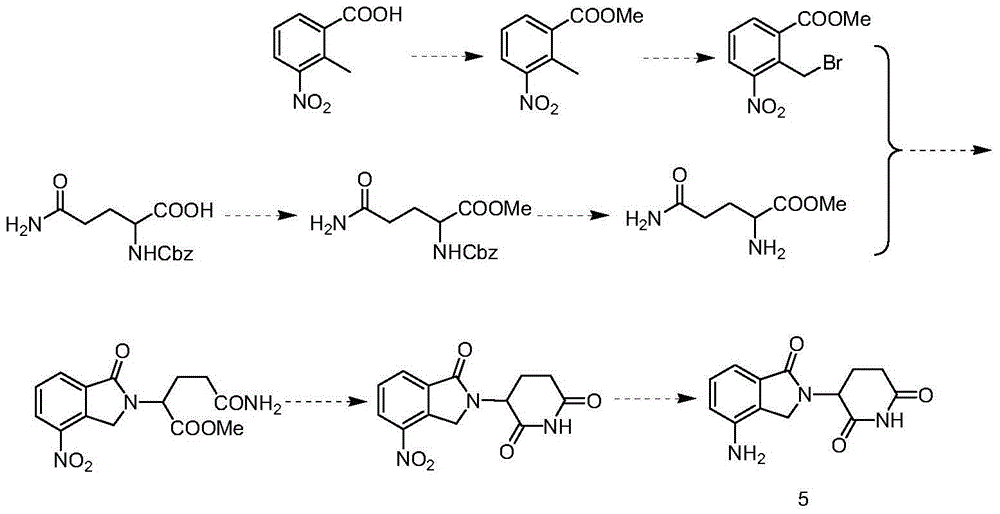
Novel preparation method of lenalidomide
A technology of lenalidomide and reflux temperature, which is applied in the field of new preparation of chemical drugs, can solve the problems of high industrialization cost, environmental pollution, and long route, and achieve the effects of low production cost, environmental friendliness, and high total reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of 3-bromo-2,6-piperidinedione
[0030] Add 10.2 g of glutarimide to 20 ml of acetic acid, add 4.5 ml of bromine, heat to reflux, stir for 3 hours, cool to room temperature, remove excess acetic acid and bromine under reduced pressure, and add the residue to chloroform and Extracted in water, the organic phase was dried, concentrated, and recrystallized from ethanol to obtain 17.1 g of white solid compound 3 (yield 99%), 1H-NMR (CDCl3, 400MHz) δ: 8.34 (s, 1H), 4.64 (t, 1H ),2.91-3.00(m,1H),2.66-2.73(m,1H),2.28-2.47(m,2H).
[0031] (2) 3-(4-nitro-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
[0032] 8.9g 4-nitro-isoindoline-1-one and 9.5g compound 3 were added to 150ml N,N'-dimethylformamide, and then 13.8g K 2 CO 3 , stirred and heated to reflux, reacted for 8 hours, stopped heating, cooled to room temperature, added 1000ml of water, stirred thoroughly, then added 300ml of ethyl acetate, extracted and separated, collected the organic phase,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com