Novel method for preparing cinnoline derivative midbody dichloro dimethyl benzoquinone
A technology of dichlorodimethylbenzoquinone and dimethylbenzoquinone, which is applied in the field of new preparation of cinnoline derivative intermediates, can solve the problems of serious environmental pollution, low yield, waste acid water, etc., and achieve reaction The effect of high total yield, environmental friendliness and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
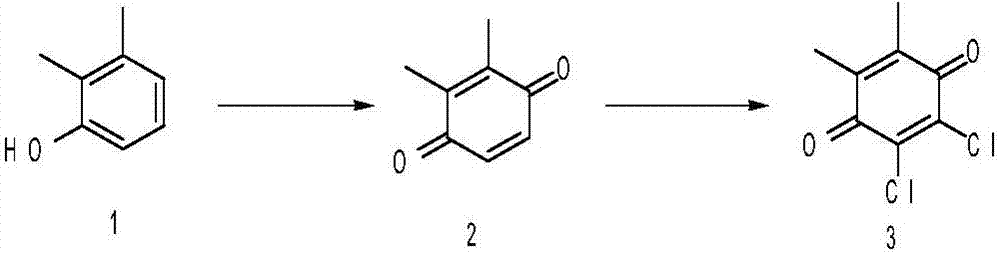
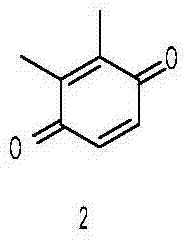
[0020] (1) Preparation of dimethylbenzoquinone
[0021] Add 24.4g of 2,3-xylenol to 150ml of acetone, slowly add 60ml of 30% hydrogen peroxide dropwise, stir at room temperature for 24 hours, concentrate under reduced pressure to remove acetone, add ethyl acetate and water, carry out extraction and separation, collect the organic phase, and dry , concentrated, and the residue was recrystallized from ethyl acetate-petroleum ether (volume ratio 1:2) to obtain 22.6 g of compound 2 as a yellow solid (yield 83.08%).
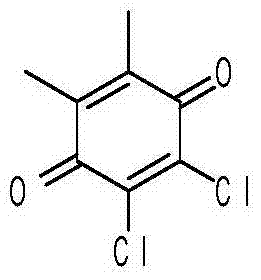
[0022] (2) Preparation of Dichlorodimethylbenzoquinone
[0023] Dissolve 22g of dimethylbenzoquinone in 200ml of acetic acid, stir, heat to reflux, feed chlorine gas, react for 5 hours, stop the reaction, remove most of the acetic acid under reduced pressure, then add ethyl acetate and water for extraction and liquid separation, The organic phase was collected, dried, concentrated, and the residue was separated on a silica gel column to obtain 29 g of dichlorodimethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com