Application of urushiol-silicon polymer in preparation of medicine for inhibiting Smad3 phosphorylation
A compound and phosphorylation technology, applied in active ingredients of hydroxyl compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: the preparation of urushiol compound of the present invention
[0025] The present invention refers to the method described in the aforementioned Chinese patent 201010149042.3 to prepare an urushiol compound, code-named GQ-5.
[0026] Preparation method: take dry resin from Anacardiaceae sumac and grind it, soak and extract in 80% ethanol three times, soak and extract in acetone once, concentrate the extract under reduced pressure to form a paste, disperse with appropriate amount of water, extract with an equal amount of ethyl acetate for 3 Second, silica gel column chromatography, chloroform-methanol gradient elution, and thin-layer chromatography (10% sulfuric acid ethanol color development) as a guide to combine components: A1-11 (chloroform-methanol 98:2), B12-17 (chloroform-methanol 96:4), C18-24 (chloroform-methanol 92:8), D25-32 (chloroform-methanol 90:10). Component C was subjected to MCI Gel CHP20P (3.5×40cm, acetone-water, 50%, 60%, 70%, 80%. ...
Embodiment 2
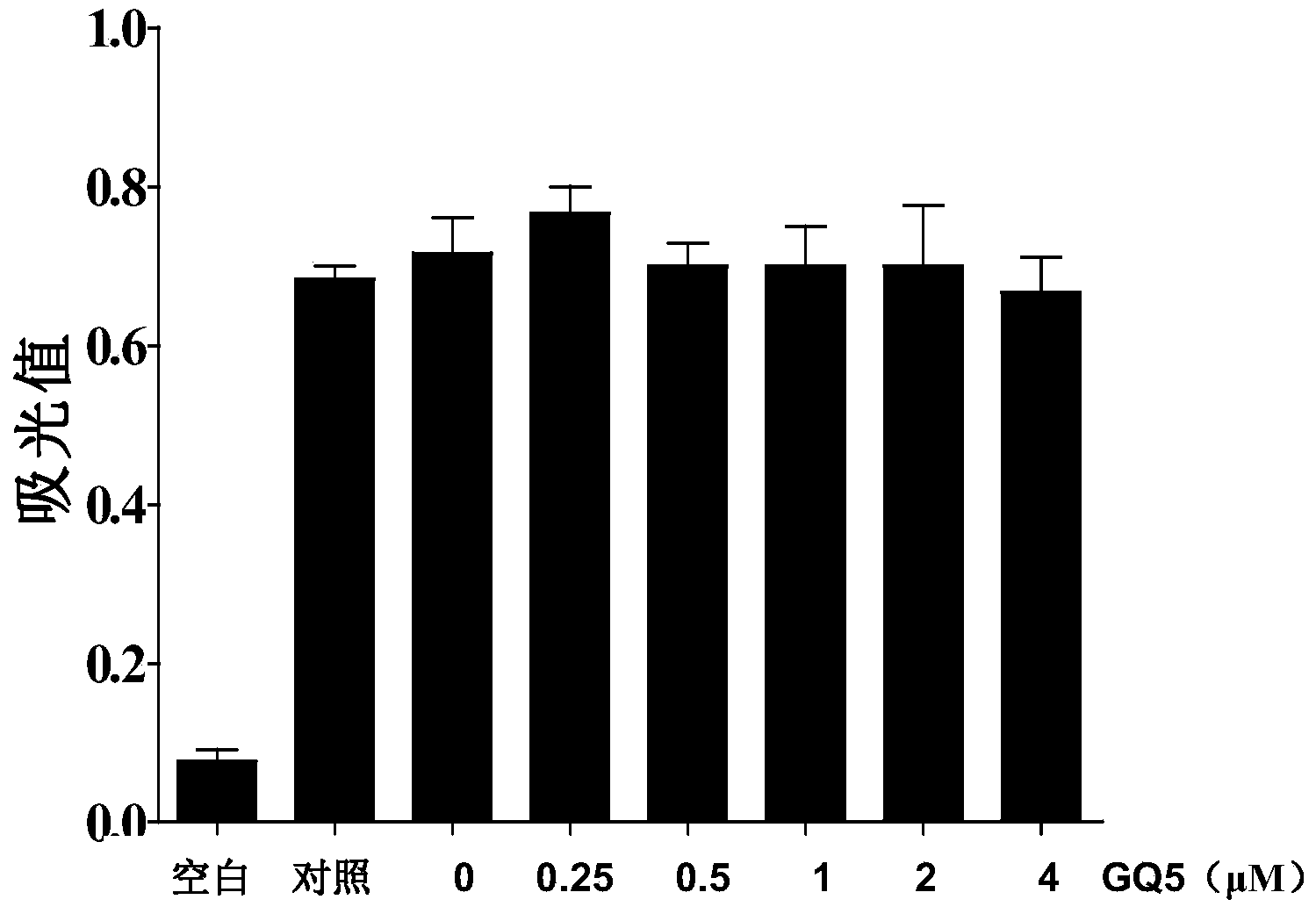
[0028] Embodiment two: the cytotoxicity of urushiol GQ-5
[0029] 1. Experimental cells: Rat normal renal tubular epithelial cell line NRK52E cells were used. Routine culture, grouping, 96-well plates without serum for 12 hours after the intervention began.
[0030] 2. Experimental grouping and processing
[0031] 1) Normal control group: continue to culture without serum for 48 hours.
[0032] 2) Medication group: pre-incubated with 0.25, 0.5, 1.0, 2.0 and 4.0 μM GQ-5 for 1 hour, and cultured without serum for 48 hours. Three replicate wells were set up for each concentration.
[0033] 3. Experimental process
[0034] GQ-5 was dissolved in DMSO to different final concentrations. After stimulation according to the above conditions, 20 μL of MTT (0.01M) was added to each well, incubated for 4 hours, the medium was discarded, 150 μL of dimethyl sulfoxide (DMSO) was added dropwise to each well, shaken at 37°C for 15 minutes, and the microplate reader was Absorbance was measur...
Embodiment 3
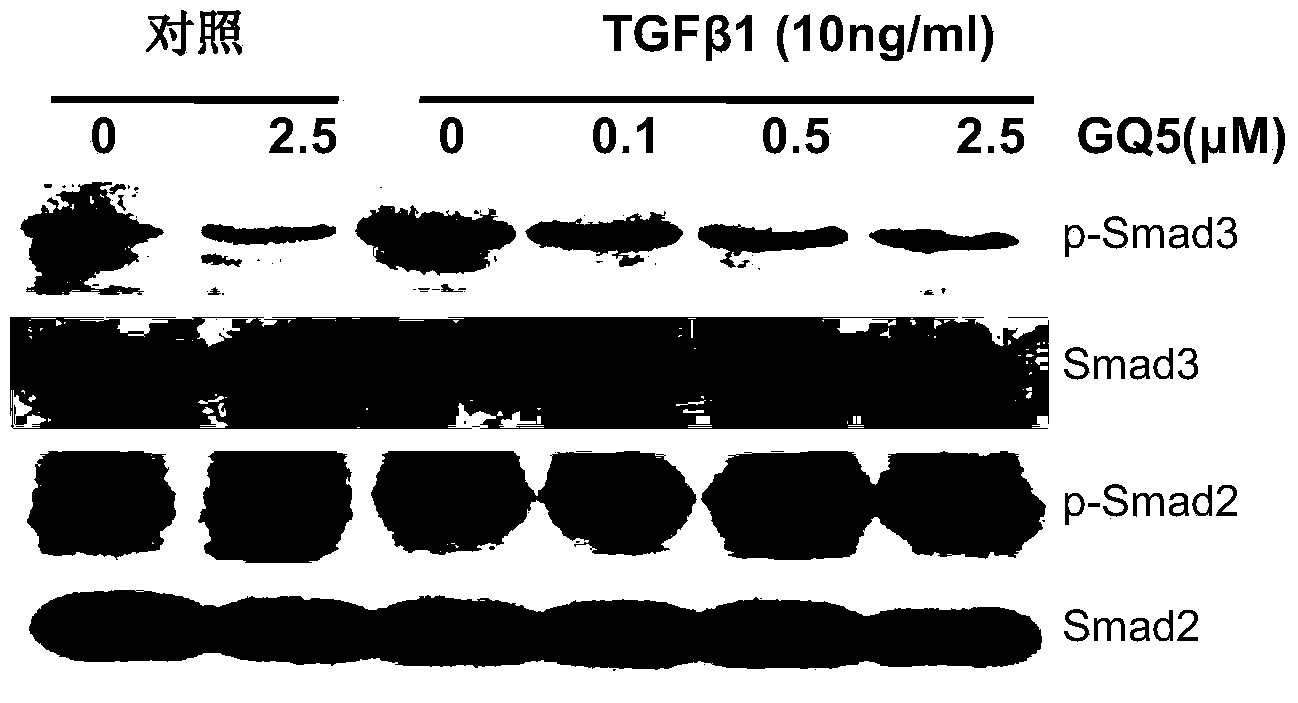
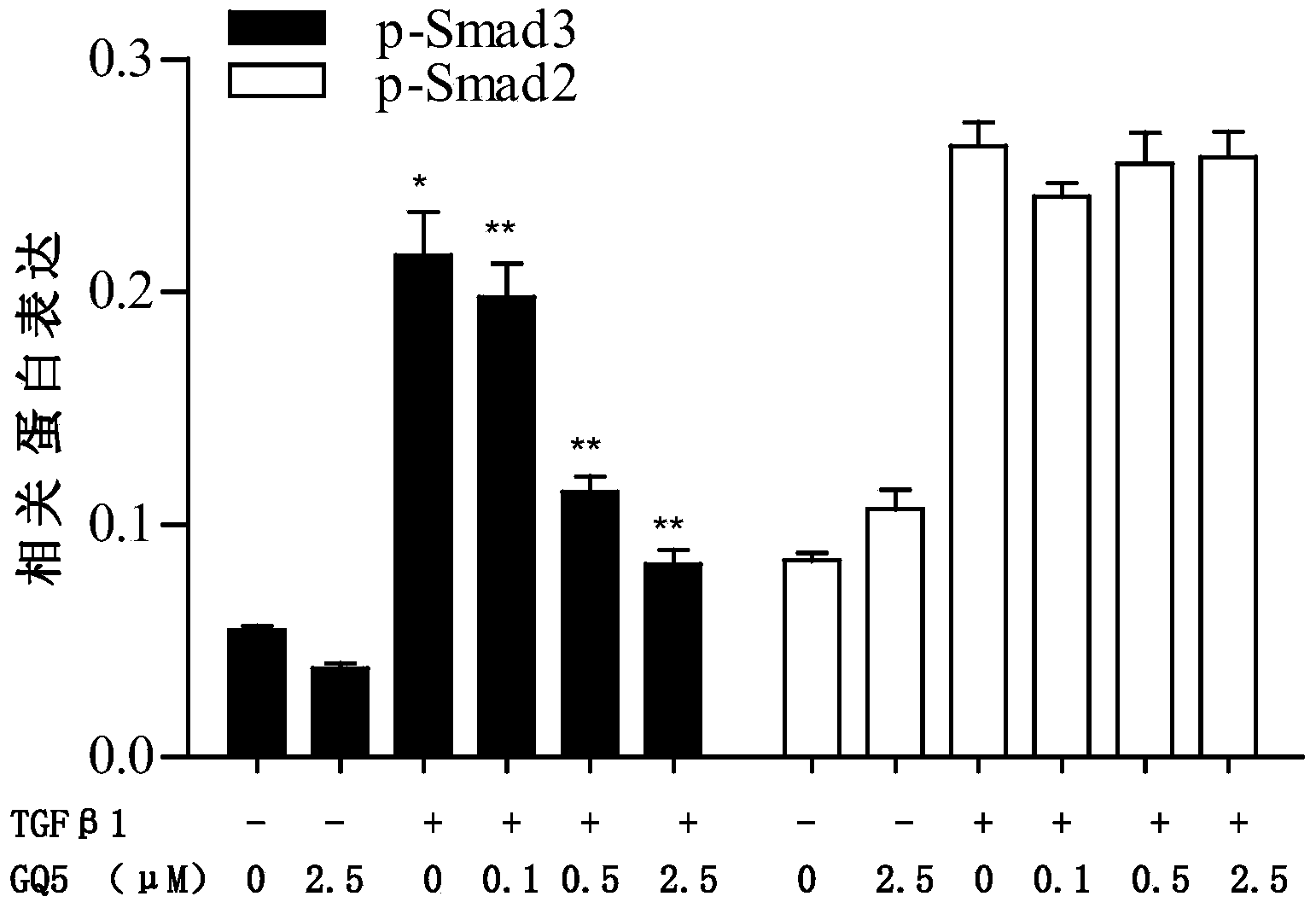
[0037] Example 3: Inhibition of urushiol GQ-5 on TGFβ1-mediated phosphorylation of Smad3
[0038] 1. Experimental cells: Rat normal renal tubular epithelial cell line NRK52E cells and rat normal fibroblast cell line NRK49F cells were used respectively. Intervention began after routine culture, grouping, and serum-free resting for 12 hours.
[0039] 2. Experimental grouping and processing
[0040] 1) Normal control group: continue culturing without serum for 2 hours.
[0041] 2) Negative control group: pre-incubated with 2.5 μM GQ-5 for 1 hour, stimulated with TGFβ 110 ng / ml for 1 hour.
[0042] 3) Positive control group: pre-incubated with 0.1% DMSO for 1 hour, stimulated with TGFβ 110ng / ml for 1 hour.
[0043] 4) Medication group: Pre-incubated with 0.1, 0.5 and 2.5 μM GQ-5 for 1 hour, stimulated with TGFβ 110 ng / ml for 1 hour.
[0044] 3. Experimental process
[0045] GQ-5 was dissolved in DMSO to different final concentrations. After stimulation according to the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com