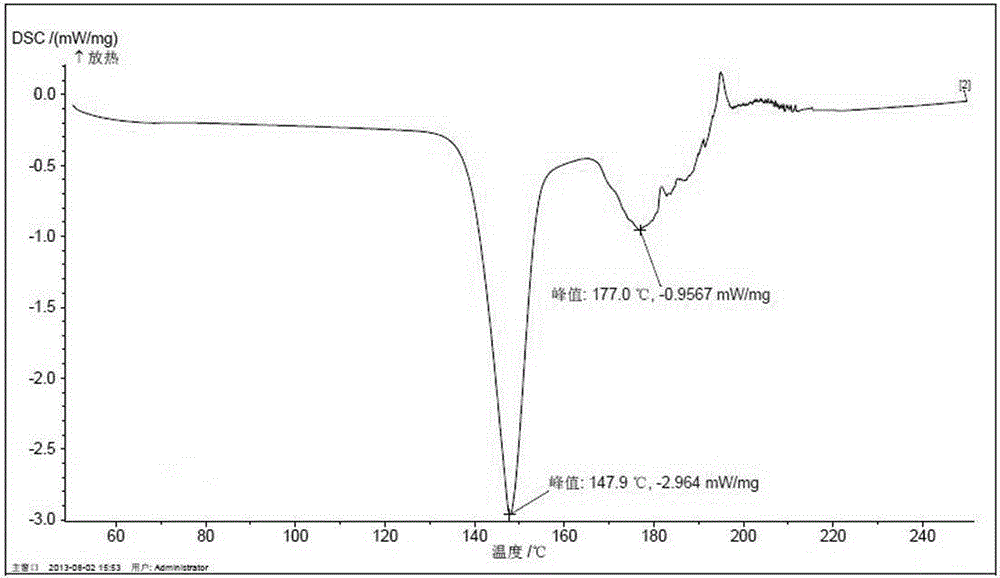
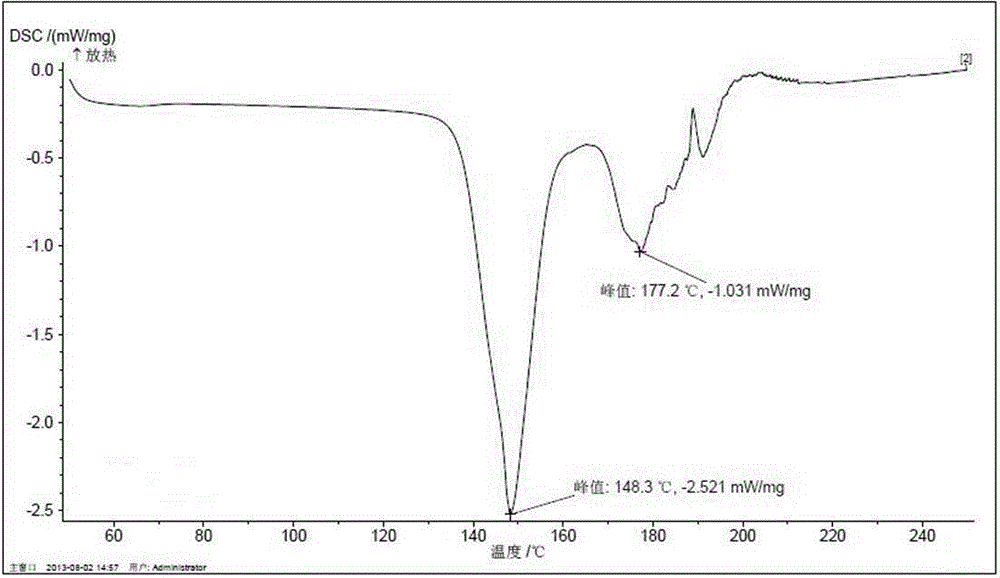
A kind of preparation method of hypotonic oral rehydration salt
A technology for rehydration salts and prescriptions is applied in the field of preparation of hypotonic oral rehydration salts, which can solve the problems affecting the sub-packaging of medicines, inaccurate filling amounts, caking of sodium chloride, etc., and achieve the effect of fast and smooth mixing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Prescription: 0.65Kg sodium chloride, 0.375Kg potassium chloride, 0.725Kg sodium citrate, 3.375Kg anhydrous glucose.
[0027] Preparation:
[0028] (1) Pulverize anhydrous glucose with 20% prescription amount together with sodium chloride, potassium chloride and sodium citrate, and pass through a 100-mesh sieve;
[0029] (2) Crush the remaining anhydrous glucose and pass through a 100-mesh sieve;
[0030] (3) Mix the mixture in step (1) with the anhydrous glucose in step (2) evenly, and take samples to test the content uniformity of intermediates;
[0031] (4) Divide according to the marked amount of 5.125g / bag to obtain the product.
[0032] Process control of mixing uniformity in step (3):
[0033] Potassium chloride has the lowest proportion in the total blend material, which is 7.32%. Therefore, it is judged whether the material is evenly mixed by testing the content uniformity of potassium chloride. The method for checking the uniformity of potassium chloride c...
Embodiment 2
[0036] Prescription: 0.65Kg sodium chloride, 0.375Kg potassium chloride, 0.725Kg sodium citrate, 3.375Kg anhydrous glucose.
[0037] Preparation:
[0038] (1) Pulverize anhydrous glucose with 10% prescription amount and sodium chloride, and pass through a 100-mesh sieve;
[0039] (2) Grind 20% of the prescription amount of anhydrous glucose with potassium chloride and sodium citrate, and pass through a 100-mesh sieve
[0040] (3) Crush the remaining anhydrous glucose and pass through a 100-mesh sieve;
[0041] (4) Mix the mixture in steps (1), (2) and the anhydrous glucose in step (3) together evenly, and take samples to test the content uniformity of intermediates;
[0042] (5) Divide according to the marked amount of 5.125g / bag, and get ready.
Embodiment 3
[0044] Prescription: 0.65Kg sodium chloride, 0.375Kg potassium chloride, 0.725Kg sodium citrate, 3.375Kg anhydrous glucose.
[0045] Preparation:
[0046] (1) Grinding 20% of the prescription amount of anhydrous glucose with sodium chloride and potassium chloride, and passing through a 100-mesh sieve;
[0047] (2) Grind anhydrous glucose with 10% prescription amount and sodium citrate together, and pass through a 100-mesh sieve
[0048] (3) Crush the remaining anhydrous glucose and pass through a 100-mesh sieve;
[0049] (4) Mix the mixture in steps (1), (2) and the anhydrous glucose in step (3) together evenly, and take samples to test the content uniformity of intermediates;
[0050] (5) Divide according to the marked amount of 5.125g / bag, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com