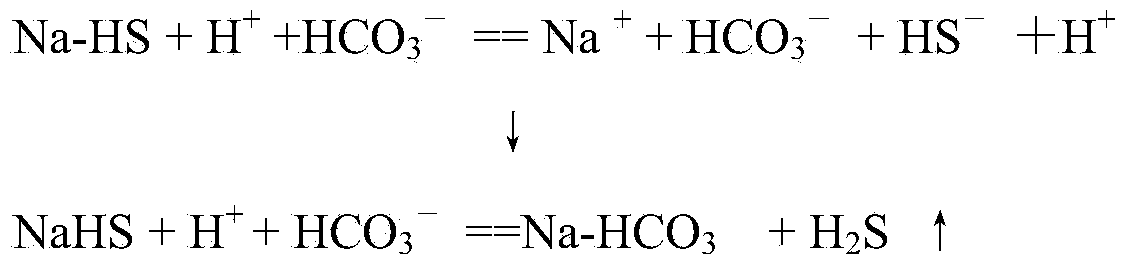Method for removing carbon dioxide from hydrogen sulfide acid gas by using buffer solution
A technology for hydrogen sulfide and carbon dioxide, which is applied in chemical instruments and methods, separation methods, and chemical separation, etc., can solve the problems of inability to cleanly separate hydrogen sulfide and carbon dioxide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
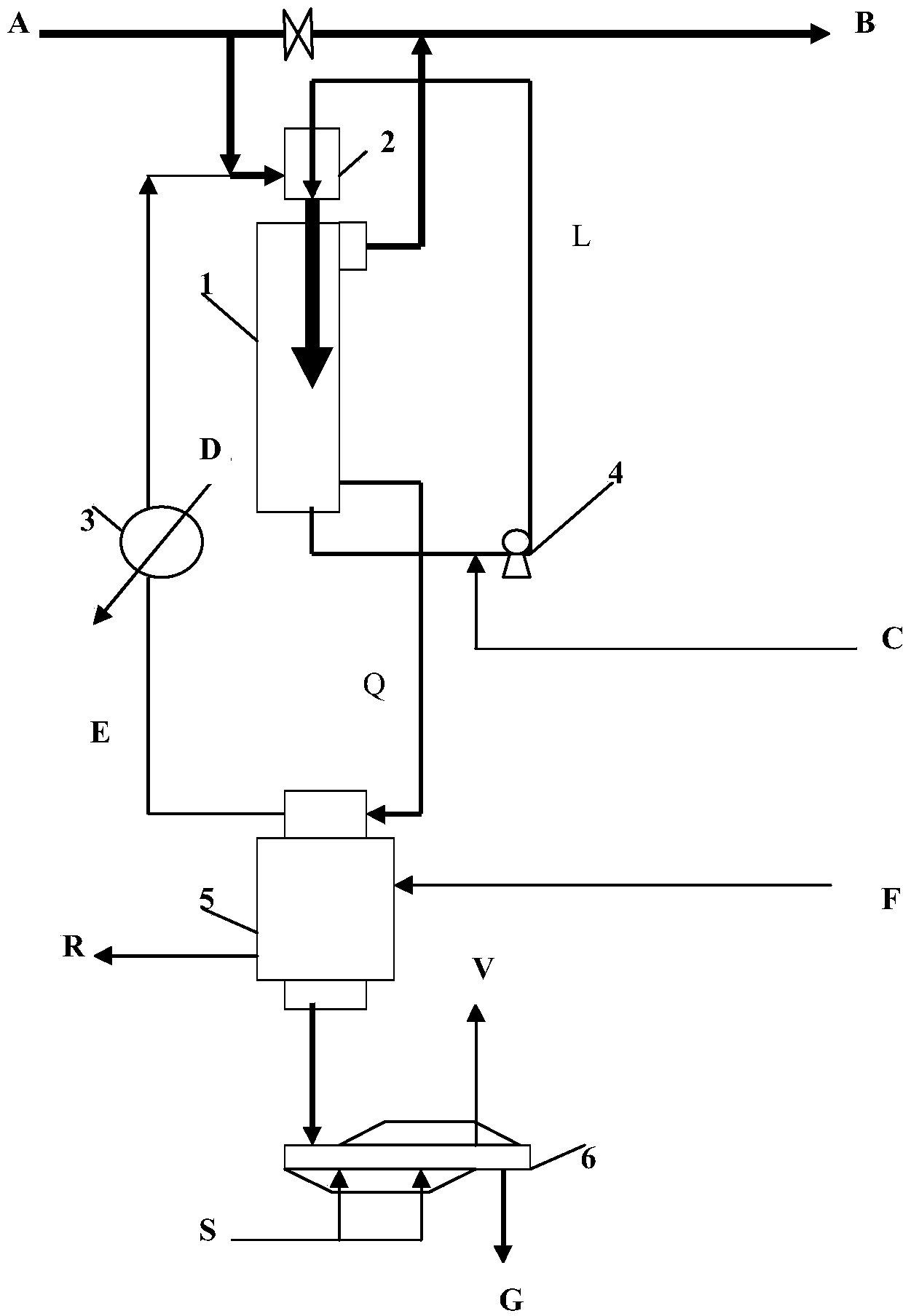
[0029] The specific operation of the method of the present invention see figure 1As shown, the non-decarbonated gas A is sprayed and inhaled by the decarburized circulating lye L in the tubular mixing reactor 2, and mixed with the lye to react to absorb carbon dioxide and part of the hydrogen sulfide in the acid gas. The lye after reacting and absorbing the acid gas enters the decarburization reaction tank 1 and stays for a sufficient time. The bicarbonate ions in the lye react with sodium bisulfide, and the sulfide ions are replaced from the sodium hydrosulfide salt molecules, and the sulfide Hydrogen produces displacement, and some hydrogen sulfide molecules leave the alkali solution and return to acid gas B after decarburization. The main component of the decarburization circulating lye is sodium bicarbonate. The pH value or free alkali concentration of the circulating lye is determined according to the amount of carbon dioxide in the acid gas. Part of the decarburized ric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com