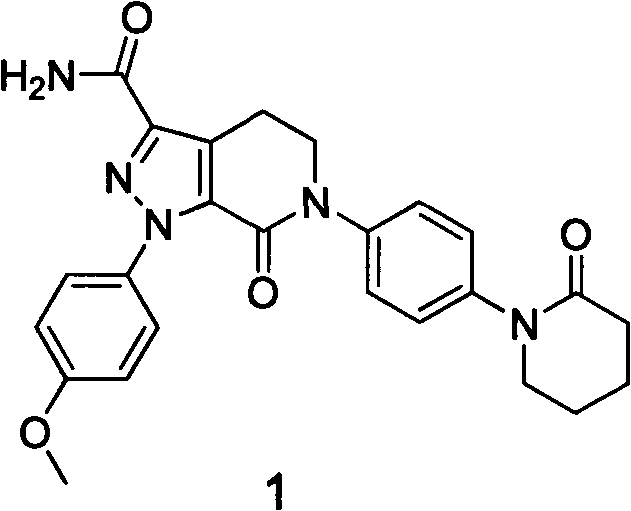
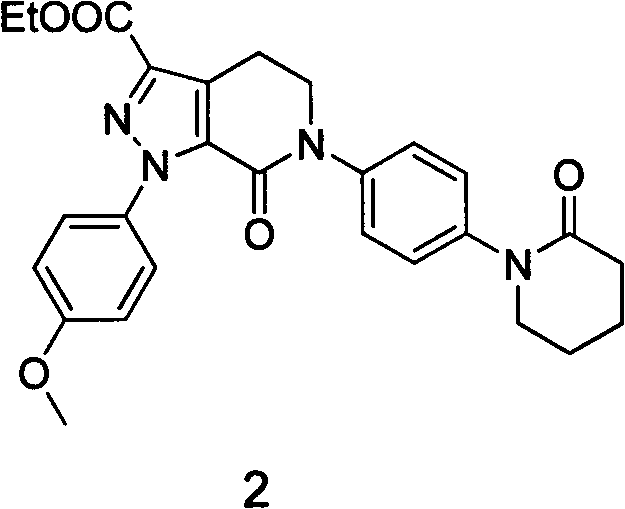
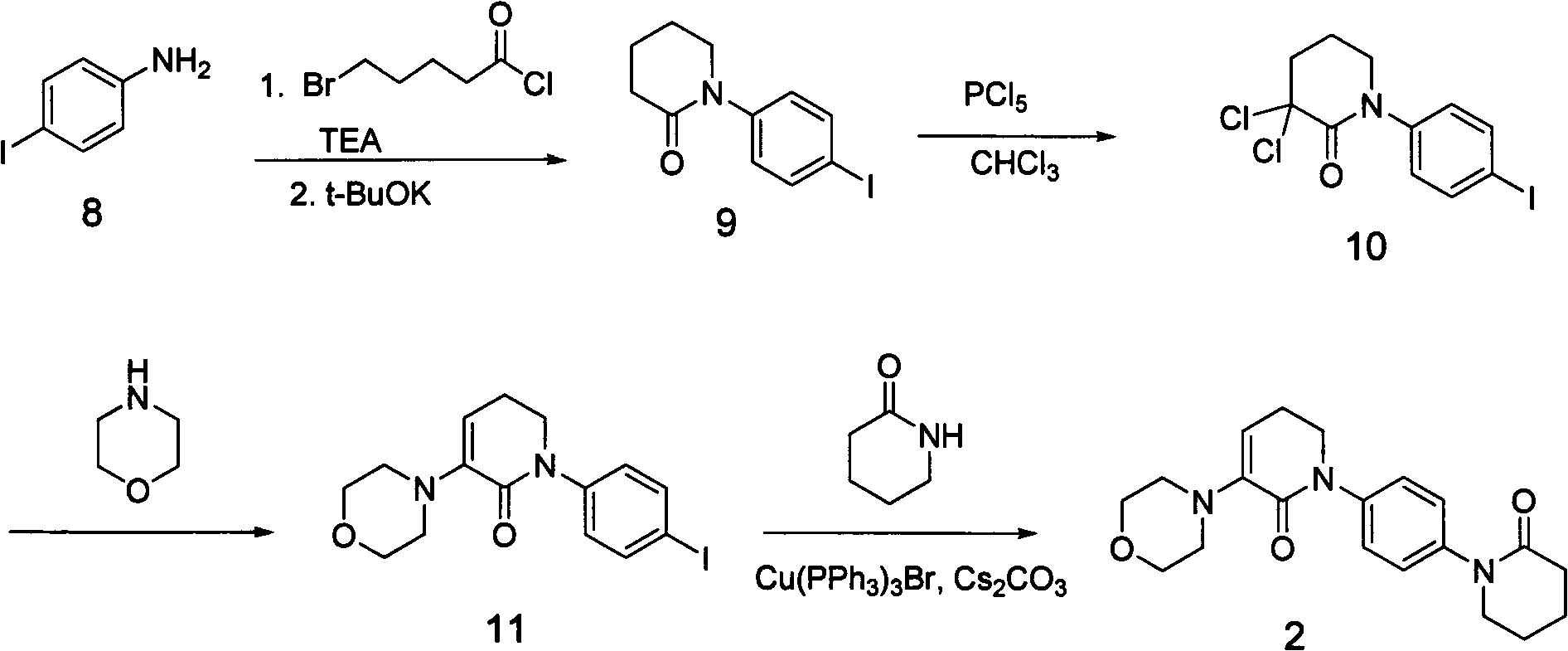
Preparation method for apixaban intermediate
A technology of intermediates and compounds, which is applied in the field of preparation of intermediates, can solve problems affecting the smooth progress of acylation reactions, and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 3-morpholin-1-[4-(2-carbonylpiperidinyl)phenyl]-5,6-dihydropyridin-2(1H)-one
[0034] first step:
[0035]
[0036] Compound 3 (24g, 0.26mol), 83ml of triethylamine, and 240ml of tetrahydrofuran were added to the reaction flask. 0°C, 5-chloropentanoyl chloride (63g, 0.4mol) was added, and reacted for 1h (the product in the reaction solution was detected as compound 3', X=Cl, MS (ESI, m / z): 212.08[M+1] ; X=Br, MS (ESI, m / z): 256.03, 258.02[M+1]); Sodium hydrogen (15.5g, 0.64mol) was added at 0°C, reacted at room temperature for 4h, evaporated THF, added dichloromethane 200ml, separated, extracted the aqueous layer with dichloromethane, washed once with saturated brine, dried over anhydrous magnesium sulfate, and spin-dried to obtain 49g of a solid, recrystallized from ethyl acetate, filtered to obtain an off-white solid, which weighed 32g after drying. The yield is 70%, and the melting point is 99-100°C.
[0037] MS (ESI, m / z): 176.11 [M+1...
Embodiment 2
[0059] Example 2: Preparation of 3-morpholin-1-[4-(2-carbonylpiperidinyl)phenyl]-5,6-dihydropyridin-2(1H)-one
[0060] The first step of acylation and cyclization reaction feeds compound 3 (10.7g, 0.12mol), triethylamine 37.2ml, 5-bromopentanoyl chloride (30g, 0.14mol), tert-butanol methyl (33.6g, 0.3mol), An off-white solid was obtained, weighing 16 g after drying. Yield 74%, melting point 98-100°C. The remaining steps were repeated in Example 1 to obtain Compound 2. Each identification data is the same as in Example 1.
Embodiment 3
[0061] Example 3: Preparation of 3-morpholin-1-[4-(2-carbonylpiperidinyl)phenyl]-5,6-dihydropyridin-2(1H)-one
[0062] The first step of acylation and cyclization reaction feeds compound 3 (6.2g, 0.07mol), 37.2ml of triethylamine, 5-bromopentanoyl chloride (30g, 0.14mol), tert-butanol methyl (33.6g, 0.3mol), Dichloromethane was used as a solvent to obtain an off-white solid weighing 9.6 g after drying. The yield is 75%, and the melting point is 98-99°C. The remaining steps were repeated in Example 1 to obtain Compound 2. Each identification data is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com