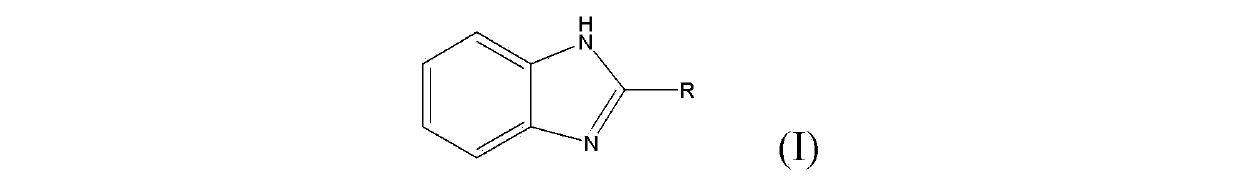
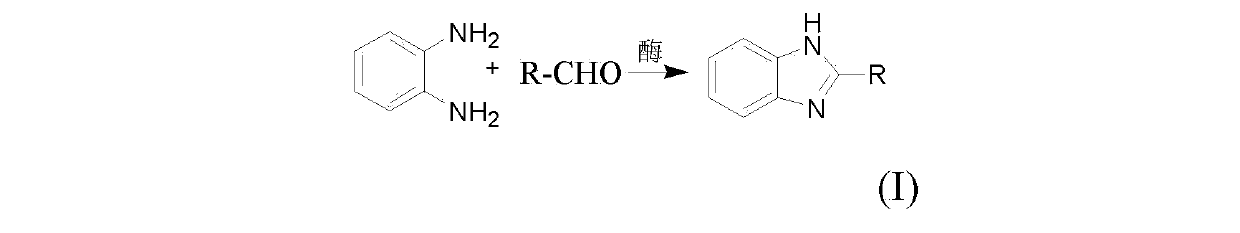
Synthetic method for 2-substitutied benzimidazole ring derivatives through enzyme catalysis
A synthesis method and technology of benzimidazole are applied in the field of synthesizing 2-substituted benzimidazole ring derivatives, which can solve the problems of complicated operation and severe reaction conditions, achieve good yield, short reaction time, and reduce acidity and alkalinity. Effects of the use of substances and metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of 2-(2-chloro-phenyl)-benzimidazole
[0023] Add about 0.5 mmol of o-phenylenediamine, about 0.5 mmol of o-chlorobenzaldehyde, 3 mL of ethanol, and about 0.035 g of lipase (Lipase AY30) into a 10 mL test tube, and shake the reaction at 35°C for about 5 hours to After the reaction was completed, the clear liquid was collected by centrifugation for precipitation, and the crude product was recrystallized from ethanol to obtain 0.11 g of the compound with a yield of 93%.
[0024] Product characterization: Mp: 275.6-278.8℃. IR(KBr) 3375,1654,1536,1520,1345. 1 H NMR (400 MHz, DMSO-d6), δ 13.02 (s, 1H), 8.30 (d, 2H, J=8 Hz), 7.75(m, 2H), 7.56 (d, 1H, J=8 Hz), 7.48(d, 1H, J=8 Hz), 7.22(m, 1H), 7.14(m, 1H). MS (EI):m / z=228.
Embodiment 2
[0025] Embodiment 2: the synthesis of 2-(2-chloro-phenyl)-benzimidazole
[0026] Add about 0.5 mmol of o-phenylenediamine, about 0.5 mmol of o-chlorobenzaldehyde, 3 mL of ethanol, and about 0.035 g of porcine pancreatic amylase (α-Amylase from hog pancreas) into a 10 mL test tube, at a temperature of 35 °C The reaction was shaken for about 5 hours to the end of the reaction, and the clear liquid was taken by centrifugation for precipitation. The crude product was recrystallized from ethanol to obtain 0.088 g of the compound, with a yield of 77%.
[0027] Product characterization: Mp: 275.6-278.8℃. IR(KBr) 3375,1654,1536,1520,1345. 1 H NMR (400 MHz, DMSO-d6), δ 13.02 (s, 1H), 8.30 (d, 2H, J=8 Hz), 7.75(m, 2H), 7.56 (d, 1H, J=8 Hz), 7.48(d, 1H, J=8 Hz), 7.22(m, 1H), 7.14(m, 1H). MS (EI):m / z=228.
Embodiment 3
[0028] Embodiment 3: the synthesis of 2-(2-chloro-phenyl)-benzimidazole
[0029] In a 10 mL test tube, add about 0.5 mmol of o-phenylenediamine, about 0.5 mmol of o-chlorobenzaldehyde, 3 mL of ethanol, and about 0.035 g of α-Amylase from Aspergillus oryzae, at a temperature of 35°C The reaction was shaken for about 5 hours to the end of the reaction, and the clear liquid was taken by centrifugation for precipitation. The crude product was recrystallized from ethanol to obtain 0.060 g of the compound with a yield of 41%.
[0030] Product characterization: Mp: 275.6-278.8℃. IR(KBr) 3375,1654,1536,1520,1345. 1 H NMR (400 MHz, DMSO-d6), δ 13.02 (s, 1H), 8.30 (d, 2H, J=8 Hz), 7.75(m, 2H), 7.56 (d, 1H, J=8 Hz), 7.48(d, 1H, J=8 Hz), 7.22(m, 1H), 7.14(m, 1H). MS (EI):m / z=228.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com