Preparation and application of a neutralizing monoclonal antibody against hepatitis C virus
A monoclonal antibody and neutralization technology, applied in the fields of molecular biology and infection immunity, can solve the problems of low eukaryotic expression yield, difficult purification, application limitations, etc., and achieve the effect of eliminating viruses and having great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
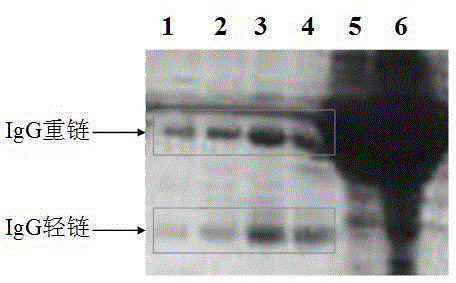
[0043] The preparation of embodiment 1 hybridoma cell line and monoclonal antibody
[0044] 1. Immunization of mice with DNA vaccine and detection of antiserum antibody titer
[0045] Using the plasmid DNA extraction kit from OMEGABiotek, pVAX-1-CpG-N2N8 was extracted, purified, and endotoxin was removed, and then different plasmids were introduced into Balb through a gene importer with an amount of 70 μg and 500 UIL-2 (immune adjuvant). / c mice (female, 6-8 weeks) were immunized in the thigh muscles on the 1st day, 10th day and 25th day respectively, and were immunized three times in total, and E2 protein (LiP, etal.Vaccine, 2007) was boosted on the 32nd day Immunization once, 60 μg / mouse, mouse serum was taken 10 days after the last booster immunization, and the antibody titer against E2 protein was detected by enzyme-linked immunosorbent assay. The mouse with the highest antibody titer was selected, and about 300 μg of E2 protein was injected intraperitoneally for 3 consec...
Embodiment 2
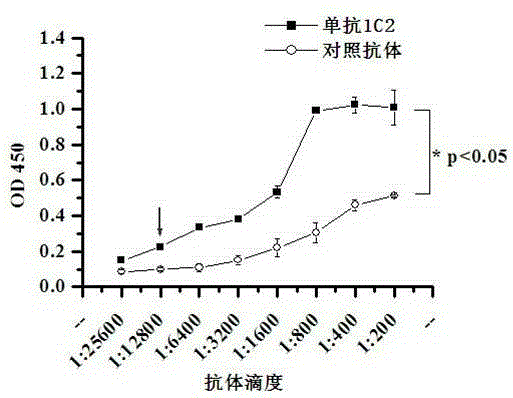
[0077] Example 2 Monoclonal Antibody Antibody Titer and Subtype Detection
[0078] The concentration of the purified monoclonal antibody 1C2 was determined to be 1.57 mg / mL, and the concentration of the control antibody was adjusted to be the same as that of 1C2. The control antibody was a monoclonal antibody that had no HCV neutralizing effect during the antibody screening process. Start with 1:200 and dilute the two antibodies (1:200, 1:400, 1:800, ... 1:25600) to detect the antibody titer of 1C2. The specific method is as follows:
[0079] (1) Coat ELISA plate with 10 μg / mL LE2 protein, 100 μL / well, overnight at 4°C.
[0080] (2) Block with 1% BSA, 37°C for 1 hour.
[0081] (3) Wash three times with PBST, 2 minutes each time. Then add the diluted 1C2 antibody and control antibody (the monoclonal antibody without HCV neutralizing effect during the antibody screening process), 100 μL / well, at 37°C for 1 hour.
[0082](4) Wash three times with PBST, 2 minutes each time. Th...
Embodiment 3
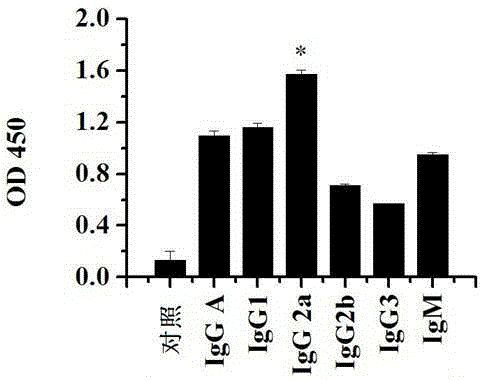
[0086] Embodiment 3 The detection of monoclonal antibody antibody specificity
[0087] In order to detect whether the monoclonal antibody 1C2 can specifically bind to the E2 protein, the ELISA method was used for the experiment, and the specific method is as follows:
[0088] (1) 10 μg / mL LE2 protein was coated on the ELISA plate, 100 μL / well, overnight at 4 °C; meanwhile, 10 μg / mL BSA and GST proteins were used as control proteins, and the ELISA plate was also coated, 100 μL / well.
[0089] (2) Block with 1% BSA, 37°C for 1 hour.
[0090] (3) Wash three times with PBST, 2 minutes each time. Then add monoclonal antibody 1C2 (1.57mg / mL) diluted 1:800, 100 μL / well, 37°C for 1 hour.
[0091] (4) Wash three times with PBST, 2 minutes each time. Then add HRP-goat anti-mouse IgG secondary antibody (1:4000) 100 μL / well, 37°C for 1 hour.
[0092] (5) Wash three times with PBST, 2 minutes each time. Add TMB for color development, 50 μL / well, then add 2M concentrated sulfuric acid t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com