Stable X-crystal-shaped agomelatine tablet and preparation method thereof
A stable technology of agomelatine tablets, applied in the field of pharmaceutical preparations, can solve the problems of inconsistent drug bioavailability, accelerated agomelatine crystal form transformation, infringement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
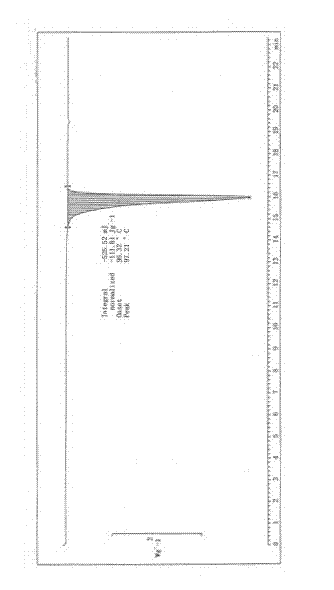
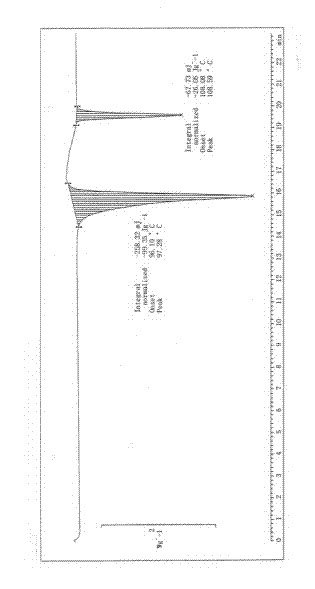
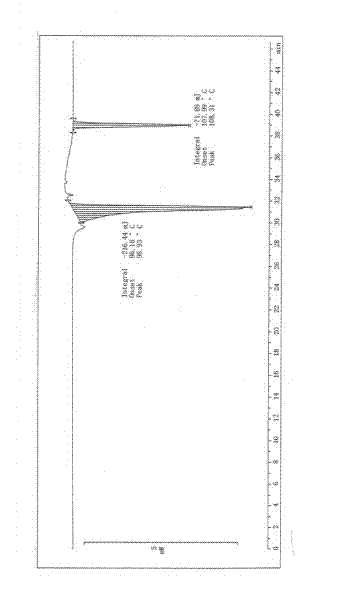
Image
Examples
Embodiment 1
[0140] Agomelatine (Form X 99%) 25g
[0141] water 20ml
[0142] Lactose 102g
[0143] Hypromellose 3g
[0144] Polyvinylpyrrolidone k30 3g
[0145] Cross-linked polyvinylpyrrolidone 13g
[0146] Magnesium Stearate 1.3g
[0147] Stearic acid 2.6g
[0148] Silica 0.3g
[0149] Process: Sift crystalline form X agomelatine according to the above weight for later use; take hydroxypropyl methylcellulose and polyvinylpyrrolidone k30 and stir in water to dissolve, let cool to room temperature, add crystalline form X agomelatine and stir homogeneous; the protective agent containing crystalline X-type agomelatine was obtained for later use; the protective agent containing crystalline X-type agomelatine was added to the mixed granulation containing lactose and part (1 / 2) cross-linked polyvinylpyrrolidone In the machine, after 2 minutes of wet granulation, granulate through a swing granulator (screen 833μm); get wet granules; dry in a fluidized bed (inlet air temperature 45°C, boi...
Embodiment 2
[0151] Agomelatine (more than 90% crystal X) 25g
[0152] water 30ml
[0153] Lactose 102g
[0154] Hydroxypropyl Cellulose 4.5g
[0155] Polyvinylpyrrolidone k30 4.5g
[0156] Croscarmellose Sodium 13g
[0157] Magnesium Stearate 1.3g
[0158] Stearic acid 2.6g
[0159] Silica 0.3g
[0160] Process: Sieve agomelatine crystal X according to the above weight for later use; take hydroxypropyl cellulose and polyvinylpyrrolidone k30 and stir in water to dissolve, let cool to room temperature, add agomelatine crystal X and stir well ; Obtain the protective agent containing crystal X-type agomelatine for later use; add the protective agent containing crystal X-type agomelatine to the mixture containing lactose and part (1 / 2) croscarmellose sodium In the granulator, after 2 minutes of wet granulation, granulate through a swing granulator (screen 833μm); get wet granules; dry in a fluidized bed (inlet air temperature 45°C, boiling bed temperature 30°C), control the moisture con...
Embodiment 3
[0162] Agomelatine (more than 85% crystal X) 25g
[0163] water 30ml
[0164] Lactose 102g
[0165] Hypromellose 4 .5g
[0166] Hydroxypropyl Cellulose 4.5g
[0167] Croscarmellose Sodium 13g
[0168] Magnesium Stearate 1.3g
[0169] Stearic acid 2.6g
[0170] Silica 0.3g
[0171]Process: Sift crystalline form X agomelatine according to the above weight for later use; take hydroxypropyl methylcellulose and polyvinylpyrrolidone k30 and stir in water to dissolve, let cool to room temperature, add crystalline form X agomelatine and stir homogeneous; the protective agent containing crystalline X-type agomelatine was obtained for later use; In the mixing granulator, after wet granulation for 2 minutes, granulate through a swing granulator (screen size 833 μm); wet granules are obtained; dry in a fluidized bed (inlet air temperature 45°C, boiling bed temperature 30°C), control the moisture content of about 2%; whole grain; calculate the yield, add other remaining excipients,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com