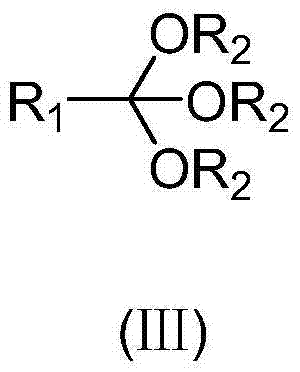
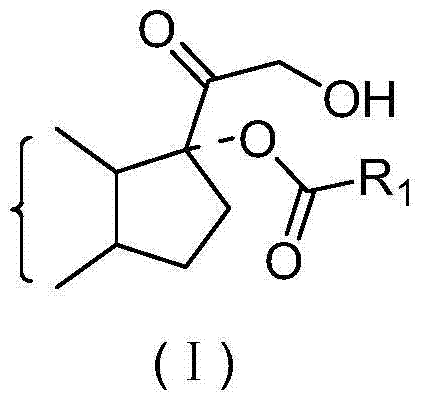
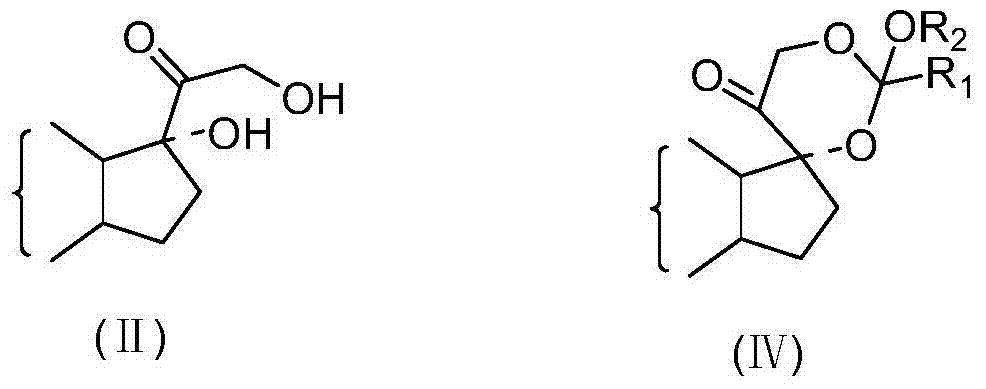Preparation method of 17 alpha-hydroxyl steroid ester
A technology of hydroxy steroid ester and hydroxy steroid is applied in the field of preparation of 17α-hydroxy steroid ester and achieves the effects of being beneficial to environmental protection, simple process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-pregna-1,4-diene-3,20-dione 50g (127.4mmol), ethyl acetate 300ml, triethyl orthopropionate Put 50 g (283.7 mmol) of the ester and 1 g of p-toluenesulfonic acid into a reaction bottle, and stir and react at 50° C. for 2.5 hours. After the reaction was completed, ethyl acetate was recovered under reduced pressure. Then add 500ml of ethanol, 8g of L-glutamic acid, 300ml of water, stir and react at 50°C for 2 hours, filter out the insoluble matter (the insoluble matter is L-glutamic acid, which can be recycled and applied), wash the filter cake with an appropriate amount of ethanol, and combine Filtrate, ethanol recovery under reduced pressure. The residue was filtered and dried to obtain 9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-pregna-1,4-diene-3,20-dione-17-propionate white powder 53.7 g, yield 94.0%.
[0039] Melting point: 230-235°C
[0040] 1 H-NMR (CDCl 3 , 400MHZ): 1.06, 1.14, 1.16, 1.30, 1.36, 1.38, 1.41, 1.55, 1.63...
Embodiment 2
[0042] According to the method of Example 1, replace 11β, 17α, 21-trihydroxypregna-4-ene-3,20-dione with 11β, 17α, 21-trihydroxypregna-4-ene-3,20-dione , reacted with triethyl orthoacetate to obtain 53.8 g of 11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione-17-acetate white powder, with a yield of 96.4%.
[0043] Melting point: 162-166°C
[0044] 1 H-NMR (CDCl 3 , 400MHZ): 1.16, 1.24, 1.26, 1.35, 1.38, 1.40, 1.41, 1.49, 1.52, 1.60, 1.63, 1.88, 1.91, 2.01, 2.29, 2.35, 2.89, 3.16, 5.85.
Embodiment 3
[0046] According to the method of Example 1, 11β, 17α, 21-trihydroxypregna-1,4-diene-3,20-dione was used to react with triethyl orthoacetate to obtain 11β, 17α, 21-trihydroxypregna -1,4-diene-3,20-dione-17-acetate white powder 55.8g, yield 96.5%.
[0047] Melting point: 162-166°C
[0048] 1 H-NMR (CDCl 3 , 400MHZ): 1.12, 1.14, 1.16, 1.35, 1.36, 1.38, 1.40, 1.41, 1.56, 1.60, 1.61, 1.87, 1.91, 2.01, 2.12, 2.29, 3.26, 4.69, 6.09, 6.28, 6.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com