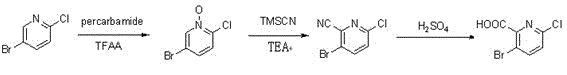Patents
Literature
92 results about "Trifluoroacetic acid anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride. Like many acid anhydrides, it may be used to introduce the corresponding trifluoroacetyl group. The corresponding acyl chloride, trifluoroacetyl chloride, is a gas, making it inconvenient to work with.
Technique of manufacturing trifluoro acetic anhydride
InactiveCN101108797AWill not polluteProcess parameters are clear and completeCarboxylic acid anhydrides preparationAcetic anhydrideGas phase
A trifluoroacetic anhydride processing technique is provided, which utilizes the trifluoroacetic acid and the phosphorus pentoxide as raw materials. The material usage rate (weight) of the trifluoroacetic acid and the phosphorus pentoxide is 1 : 1.5 to 1 : 0.65 and the phosphorus pentoxide is added twice. The entire operation process is done in the fully sealed state, the high boiling substance (after cut) and the tail gas are treated and recycled after collecting the finished products; the condensing water and the cooling water are water from deep well and the byproduct phosphoric acid are all recycled. The invention prepares the trifluoroacetic anhydride applied in the highly efficient liquid chromatogram and the derivating agent of the gas chromatogram, the solvent, catalyst, the condensation dehydration agent and the retention agent of various synthetic reaction, which has complete, canonical and reasonable process and convenient operation, therefore is suitable for industrial production, can effectively improve the purity and the yield of the products, has no environment pollution and reduces the production cost.
Owner:申厚宝
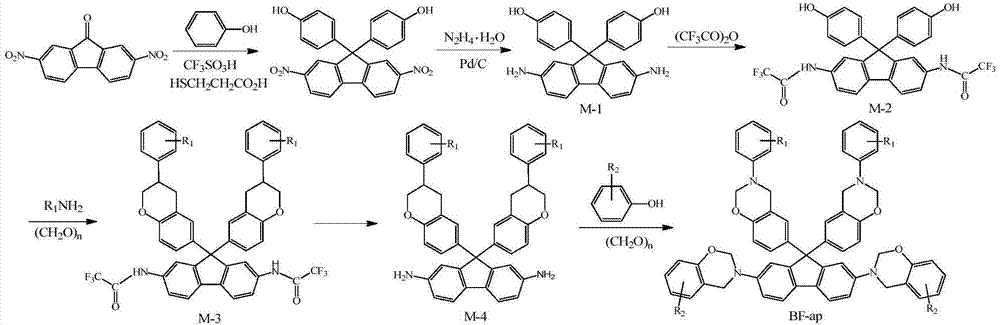
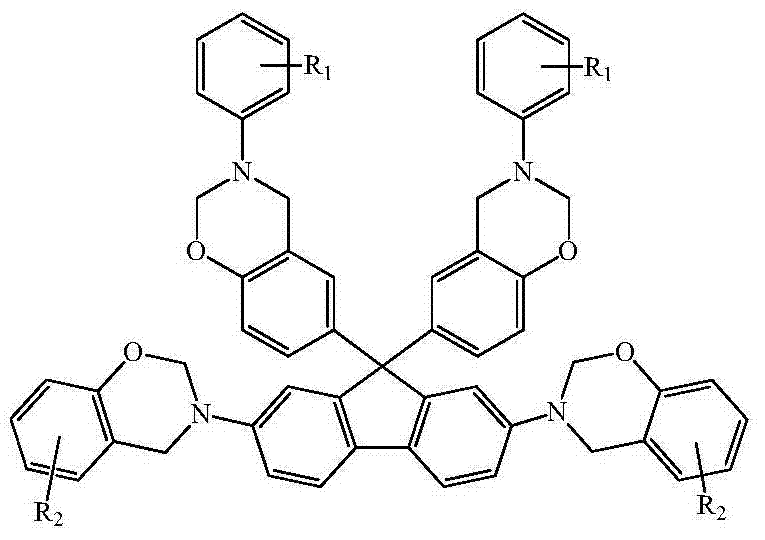
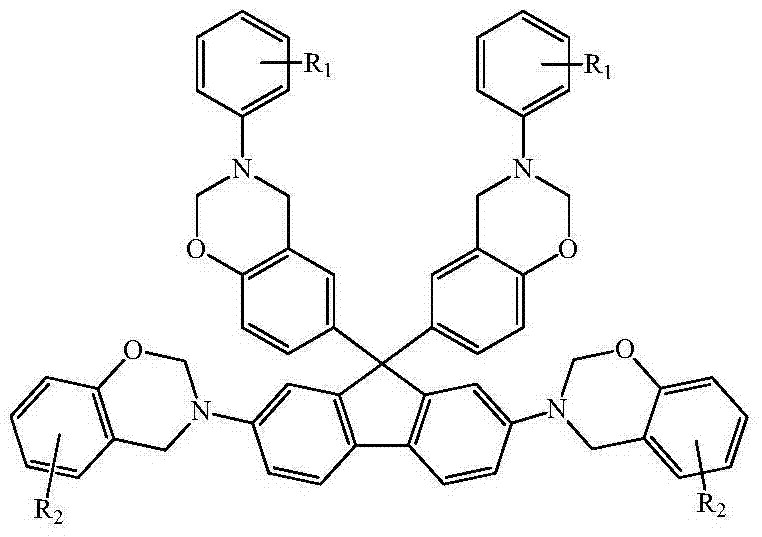
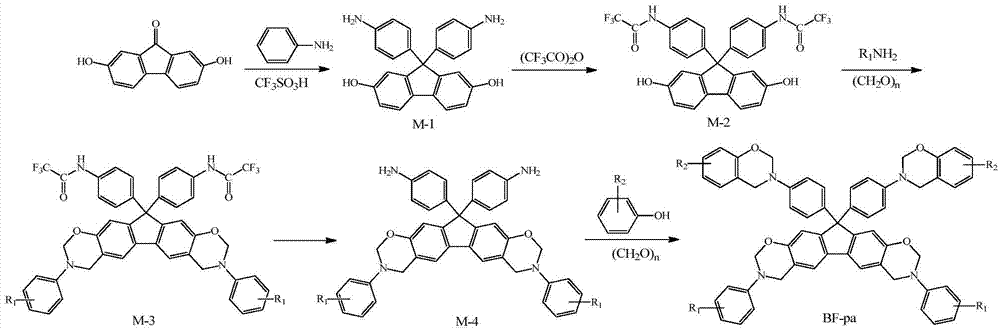
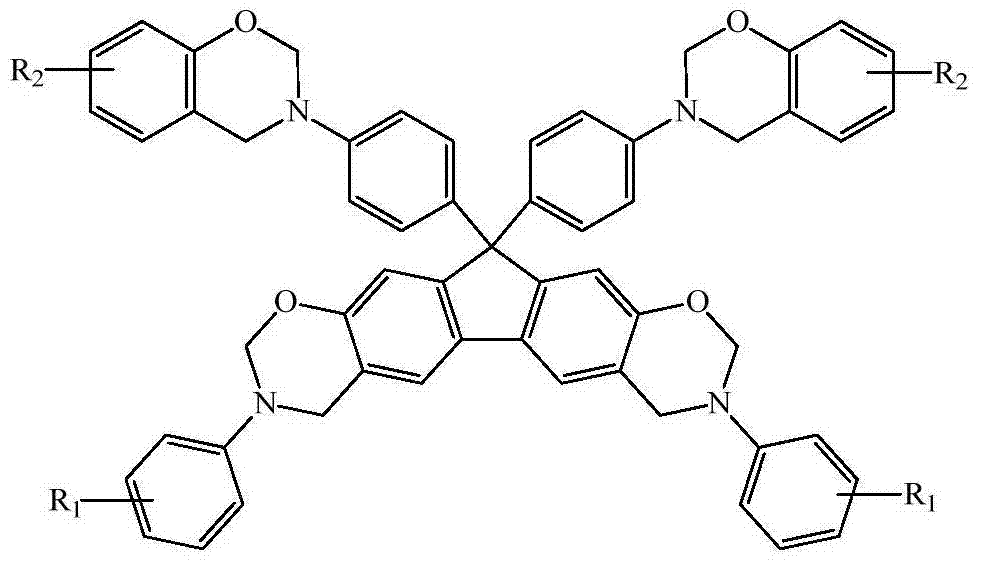
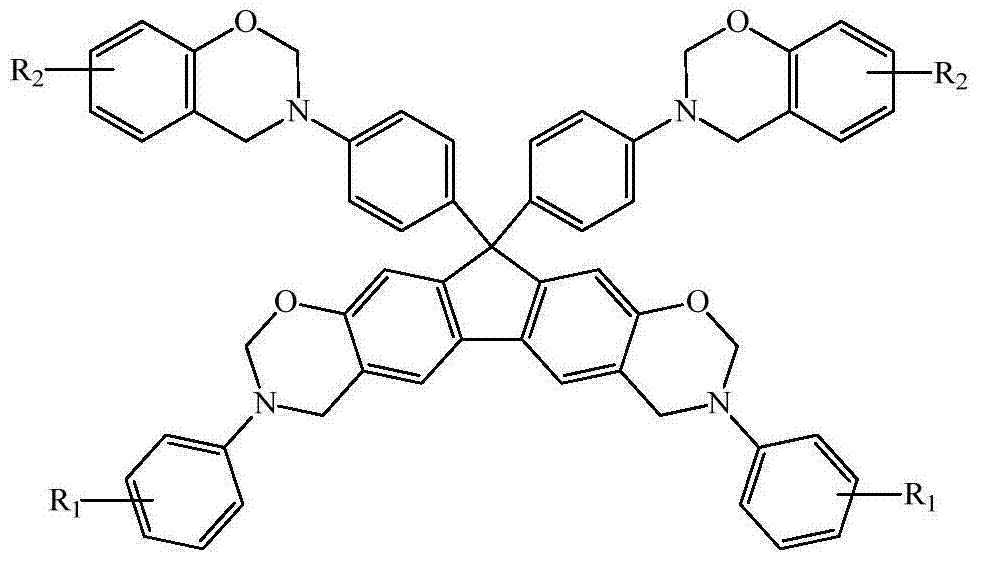
N-full-aromatic hydrocarbon diamine-bisphenol tetrafunctional fluorenyl benzoxazine and preparation method thereof
InactiveCN103896867AImprove flame retardant performanceImprove heat resistanceOrganic chemistryMannich condensationAromatic hydrocarbon
The invention provides N-full-aromatic hydrocarbon diamine-bisphenol tetrafunctional fluorenyl benzoxazine and a preparation method thereof. The method comprises the following steps: firstly, protecting amino in 2,7-diamino-9,9-bis-(4-hydroxyphenyl) fluorene by using trifluoroacetic anhydride to generate 2,7-bistrifluoroacetamido-9,9-bis(4-hydroxyphenyl) fluorene, and then carrying out Mannich condensation reaction with aromatic amine and paraformaldehyde to form a 2,7-bistrifluoroacetamido bisphenol fluorenyl benzoxazine monomer; carrying out amino deprotection, and then carrying out secondary Mannich condensation reaction with a phenolic compound and paraformaldehyde to finally obtain a novel N-full-aromatic hydrocarbon diamine-bisphenol tetrafunctional fluorenyl benzoxazine monomer. By adopting the N-full-aromatic hydrocarbon diamine-bisphenol tetrafunctional fluorenyl benzoxazine and the preparation method thereof, the problems that the fluorenyl polybenzoxazine with a large steric hindrance structure is small in molecular weight, low in crosslinking density and poor in tenacity, and the thermal performance is reduced by introducing a flexible group are solved, the processing property of the polymer is improved, and controllable structure and performance of polybenzoxazine are achieved.
Owner:HARBIN ENG UNIV
Synthesis method for pesticide intermediate trifluoroacetonitrile
InactiveCN103804231AHigh yieldEase of industrial productionPreparation by carboxylic acid amide dehydrationSynthesis methodsSilicon tetrachloride
The invention provides a synthesis method for a pesticide intermediate trifluoroacetonitrile. The synthesis method comprises the following steps: adding trifluorofluoroacetamide, carbon tetrachloride and trifluoroacetic anhydride into a gas reaction kettle; raising the temperature to 150-180 DEG C; dehydrating and reacting; and cooling to obtain the trifluoroacetonitrile. A reaction formula is as follows: CF3CONH2+PPh3+CCl4->CF3C(Gl)=NH+O=PPh3+CHCl3CF3C(Cl)=NH+PPH3->CF3CN+PPh3+HCl.
Owner:JIANGSU INST OF ECOMONES
Preparation method of 1,1,1,5,5,5-hexafluoro acetylacetone
InactiveCN102260151AReduce pollutionLow costOrganic compound preparationCarbonyl compound preparationTrifluoroacetic acidHexafluoro-acetylacetone
The invention provides a preparation method of 1,1,1,5,5,5-hexafluoro acetylacetone. The preparation method comprises the following steps: carrying out first-stage reaction on trifluoroacetyl acetate and trifluoroacetic anhydride under the action of a catalyst at 40-60 DEG C for 5-10 hours; then raising the temperature to 120-150 DEG C for second-stage reaction, and distilling out the resulting 1,1,1,5,5,5-hexafluoro acetylacetone while reacting to obtain a crude 1,1,1,5,5,5-hexafluoro acetylacetone product; and finally purifying the crude product to obtain a refined 1,1,1,5,5,5-hexafluoro acetylacetone product. In the preparation method provided by the invention, as inexpensive trifluoroacetic anhydride and trifluoroacetyl acetate are used as raw materials for the reaction, the cost of the product is dramatically reduced; and as no acid is generated in the reaction process, the environmental pollution is low.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
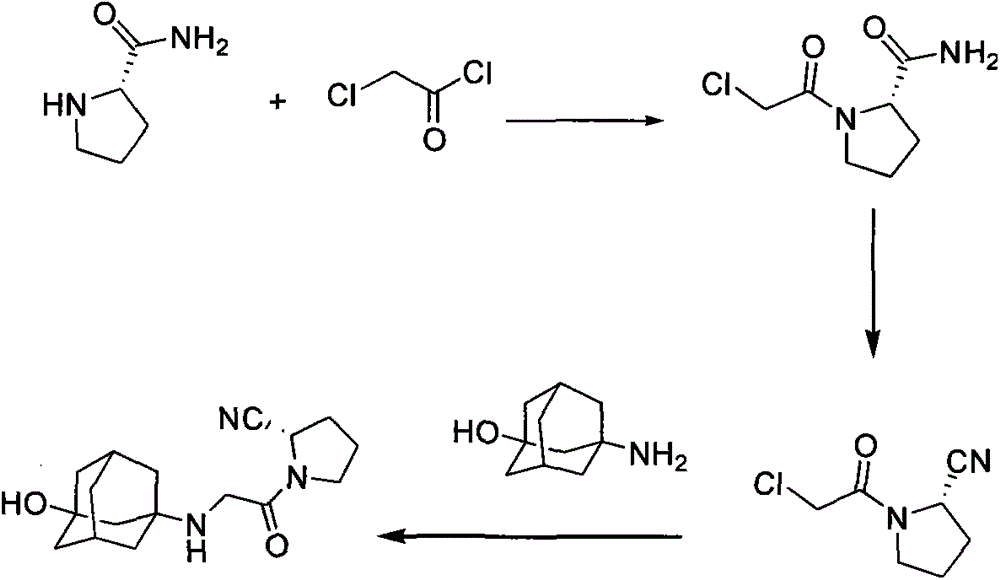
Method for preparing substituted (S)-pyrrolidine-2-formonitrile and vildagliptin
The invention provides a method for preparing (S)-1-(2-halogenated acetyl)pyrrolidine-2-formonitrile as shown in the formula (I). Optionally in the presence of a diluent, (S)-1-(2-halogenated acetyl)pyrrolidine-2-formamide and a dehydrating agent propanephosphonic acid cyclic anhydride (T3P) react with each other. The invention also provides a method for preparing vildagliptin by using S-prolinamide involving the above reaction. The method for preparing the compound as shown in the formula (I) has the following advantages: use of expensive trifluoroacetic anhydride is not required, yield is increased, and cost is reduced; use of cyanuric chloride prepared from highly toxic raw materials is not required, and the reaction is more environmentally friendly; and an improved method for preparing vildagliptin is then obtained. In the formula (I) and formula (II), X1 is halogen.
Owner:HYBIO PHARMA
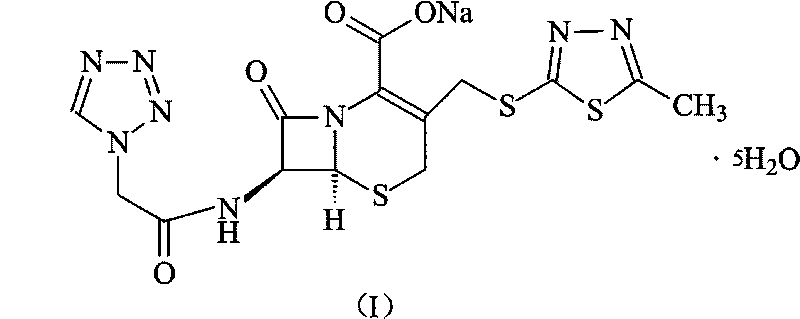
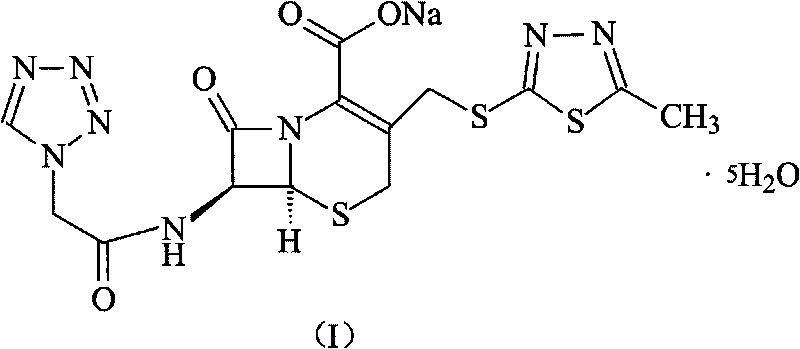
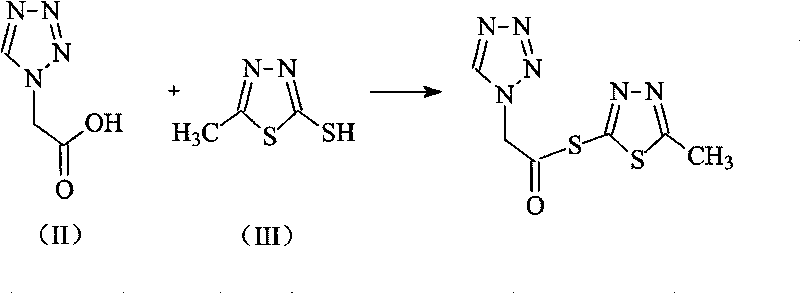
Cefazolin sodium pentahydrate compound of new route
The invention relates to a cefazolin sodium pentahydrate compound of a new route, and particularly provides a method for synthesizing the cefazolin sodium pentahydrate compound. The method comprises the following steps: synthesizing tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester by using tetrazolyl acetic acid and 2-mercapto-5-methyl-1,3,4-thiadiazole as raw materials; and generating cefazolin sodium through the reaction between the tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester and 7-aminocephalosporanic acid. Compared with that the method for synthesizing tetrazole acetic acid 2-mercapto-1,3,4-thiadiazole ester in the prior art uses expensive reagents such as trifluoroacetic anhydride, aluminium trimethide or DCC and the like as a condensation agent, the method improves the synthesis method, not only simplifies the operation steps, but also, surprisingly, greatly improves the product yield and the purity, reduces the cost, and lays a foundation for industrialization.
Owner:HAINAN MEIDA PHARMA
Method for synthetizing 2-trifluoromethyl oxazole compound
InactiveCN108033928AAdaptableSimple and fast operationOrganic chemistrySynthesis methodsTellurium compounds
The invention discloses a method for synthetizing a 2-trifluoromethyl oxazole compound. According to the method, trifluoroacetic anhydride is used as a reaction reagent; an oxime ester derivative is used as a reaction substrate; a tellurium compound and iodine are used as catalysts; in a solvent, heating is performed at 110 to 120 DEG C; stirring reaction is performed for 1 to 8h; the 2-trifluoromethyl oxazole compound is prepared. The synthesis method has the advantages that the yield is high; the raw materials can be easily obtained; the operation is simple and convenient; the functional group universality is good, and the like. Good industrial application prospects are realized.
Owner:FUZHOU UNIV
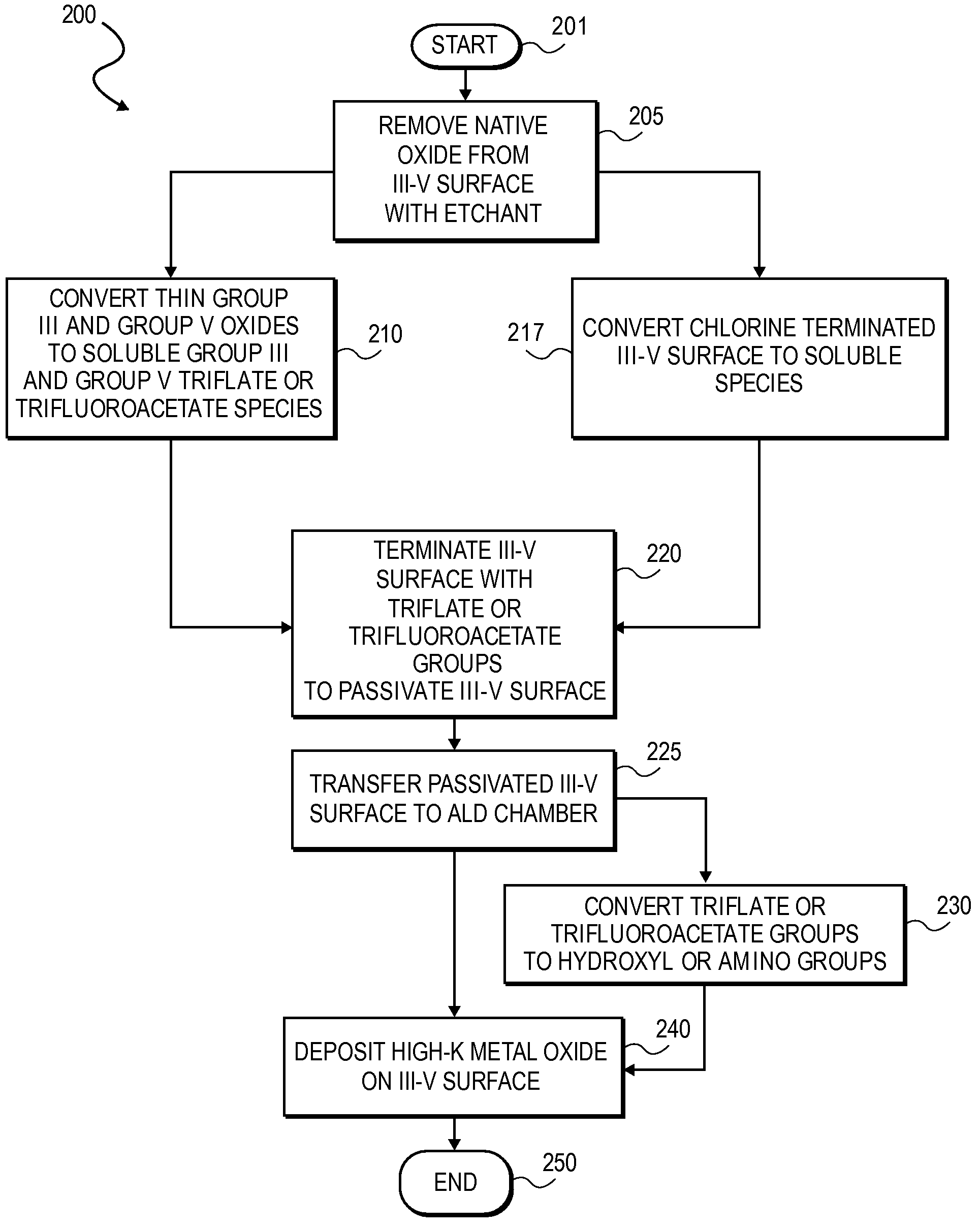
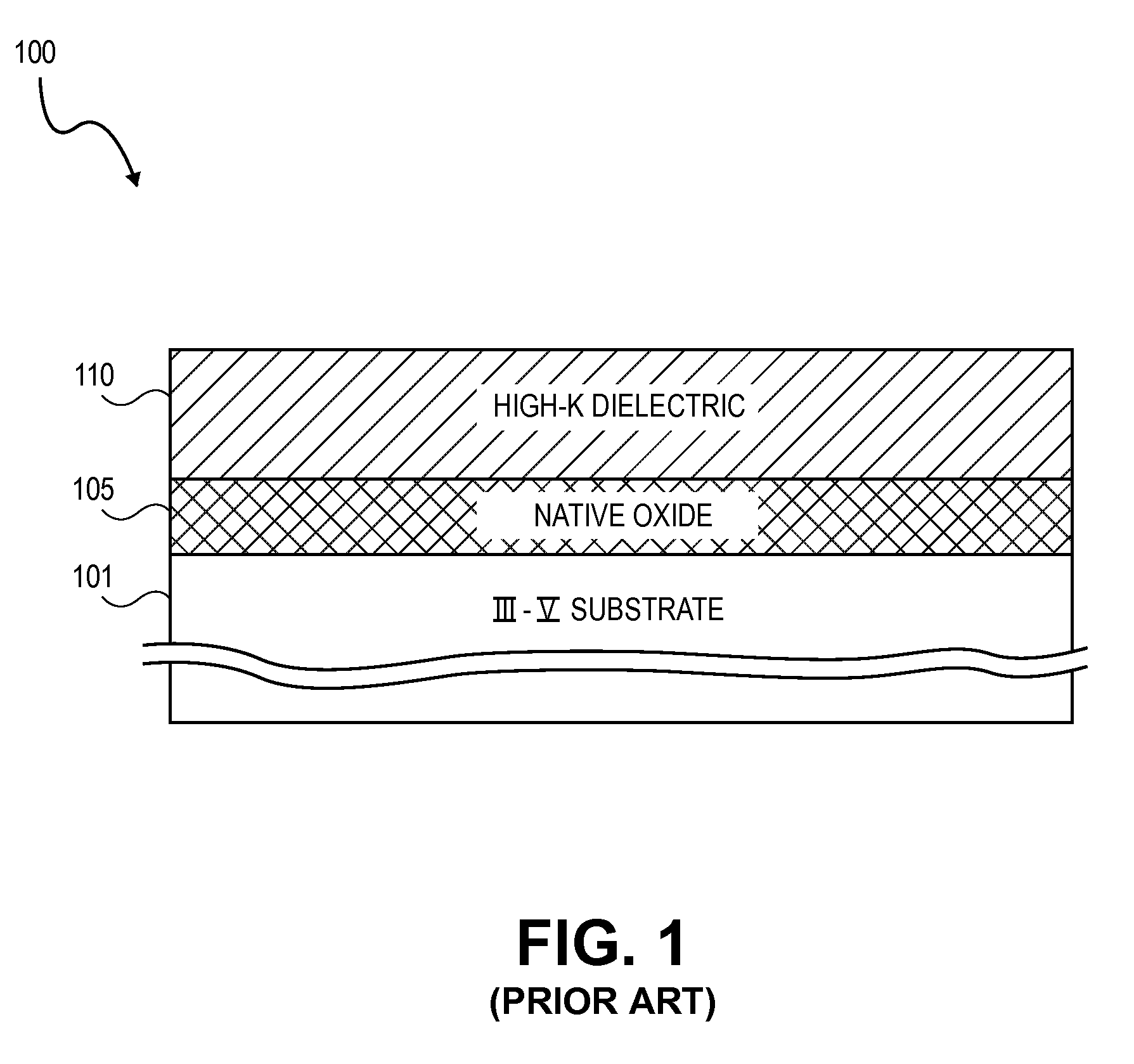
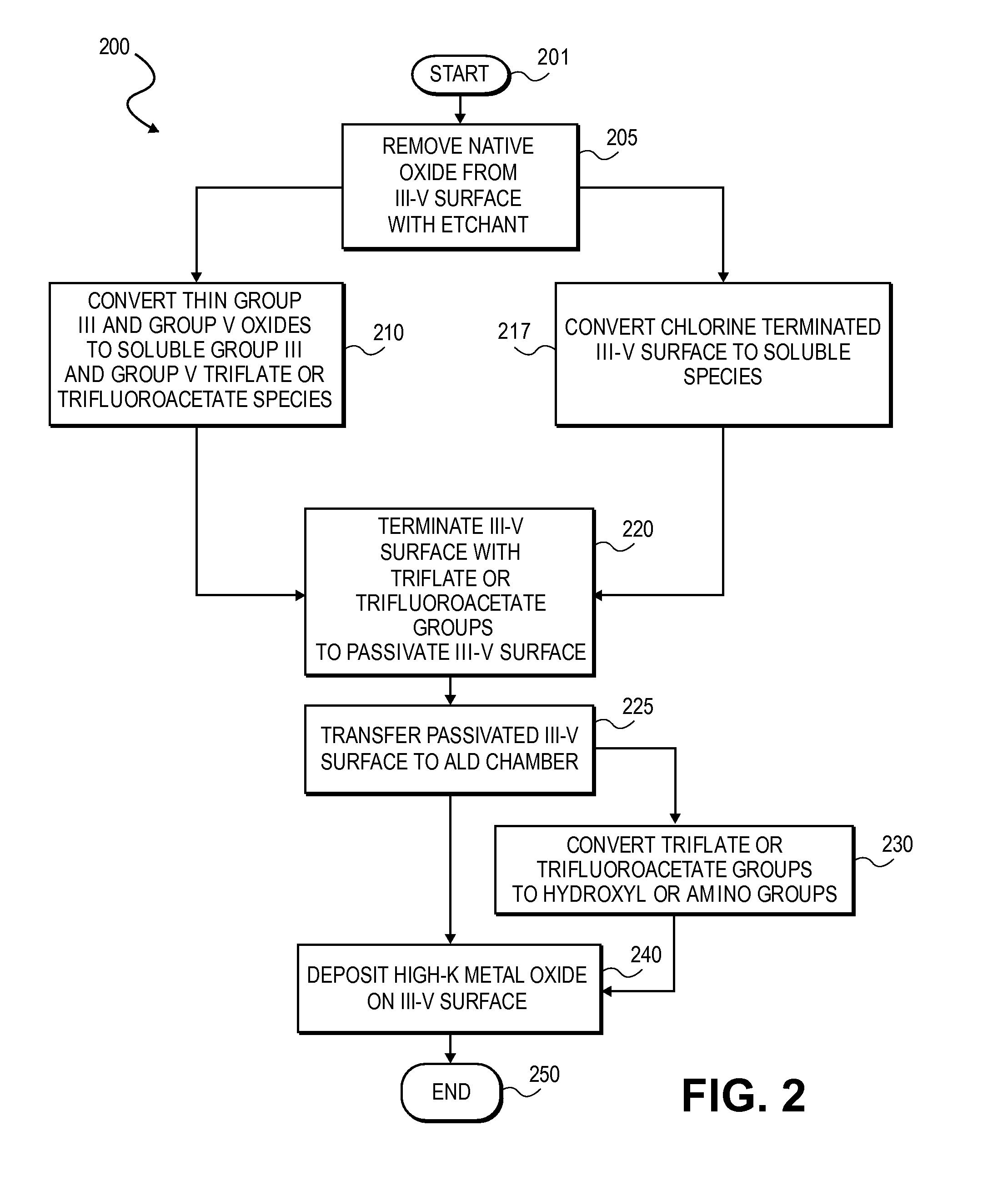
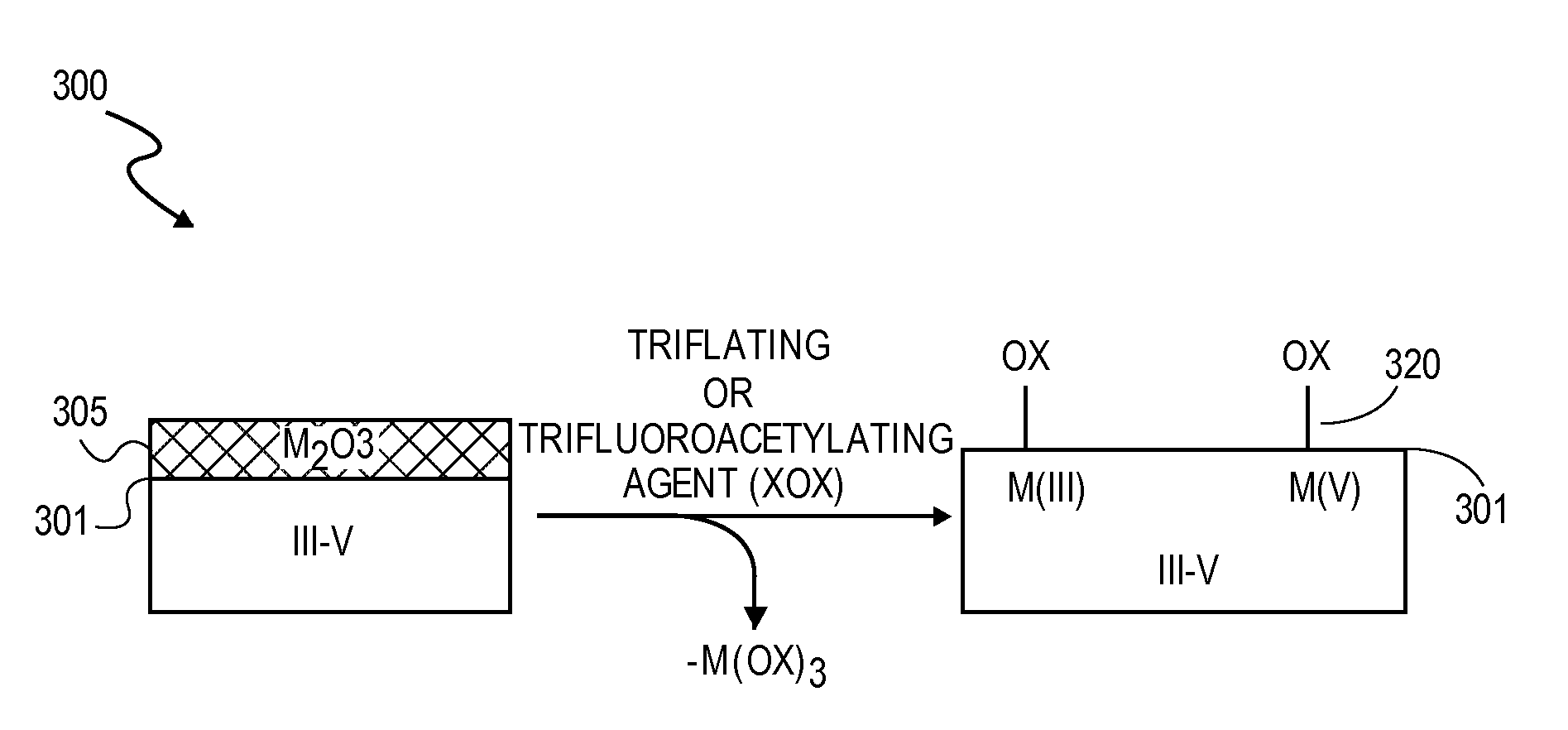
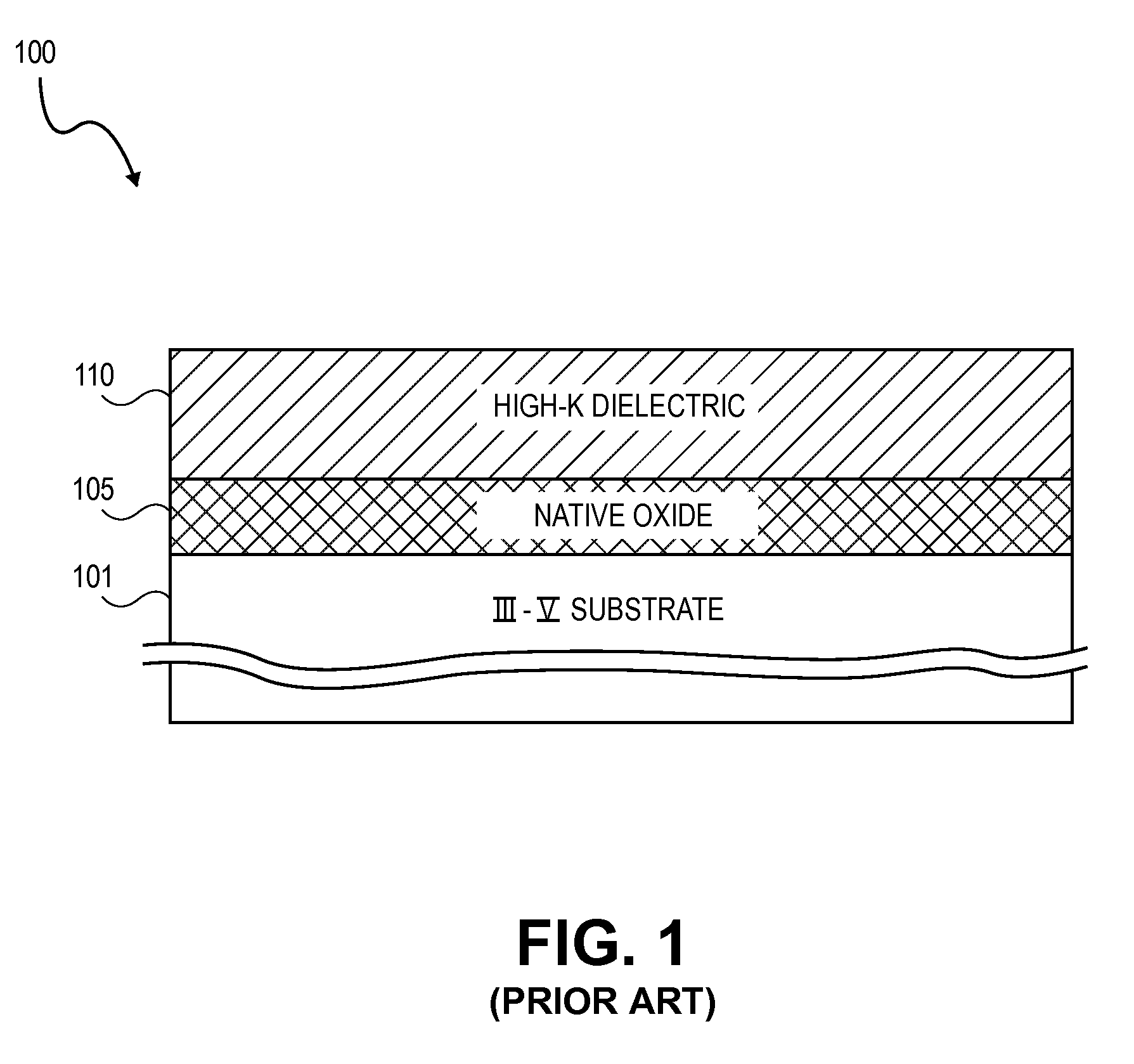
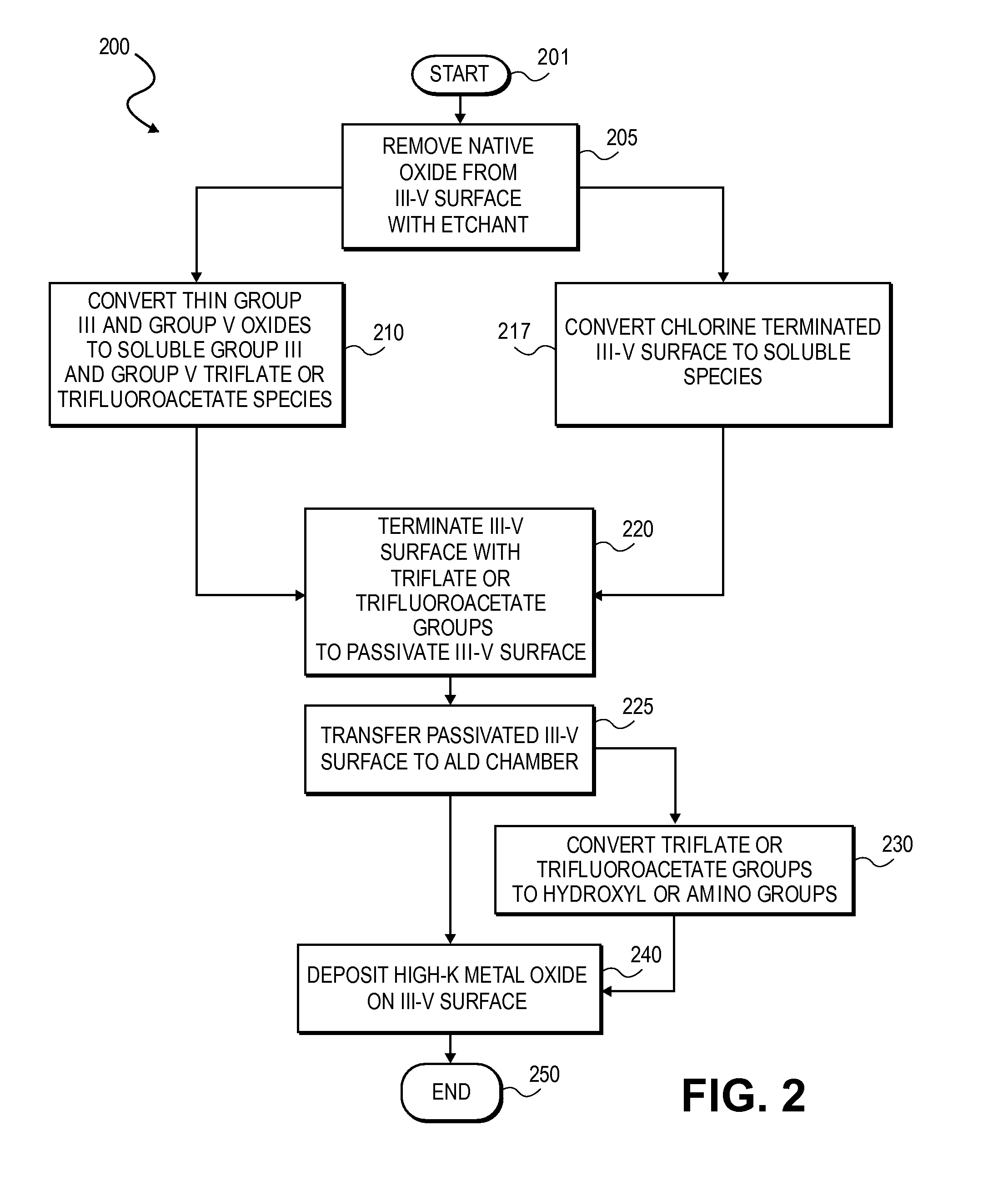
High K dielectric growth on metal triflate or trifluoroacetate terminated III-V semiconductor surfaces
InactiveUS7763317B2Polycrystalline material growthSemiconductor/solid-state device manufacturingTrifluoromethanesulfonic anhydrideTriflic acid
Surface preparation of a compound semiconductor surface, such as indium antimonide (InSb), with a triflating agent, such as triflic anhydride or a trifluoroacetylating agent, such as trifluoroacetic anhydride is described. In one embodiment, the triflating or trifluoroacetylating passivates the compound semiconductor surface by terminating the surface with triflate trifluoroacetate groups. In a further embodiment, a triflating agent or trifluoroacetylating agent is employed to first convert a thin native oxide present on a compound semiconductor surface to a soluble species. In another embodiment, the passivated compound semiconductor surface is activated in an ALD chamber by reacting the triflate or trifluoroacetate protecting groups with a protic source, such as water (H2O). Metalorganic precursors are then introduced in the ALD chamber to form a good quality interfacial layer, such as aluminum oxide (Al2O3), on the compound semiconductor surface.
Owner:INTEL CORP
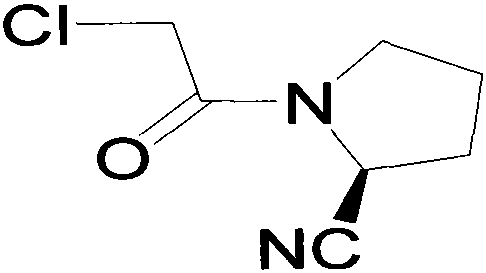
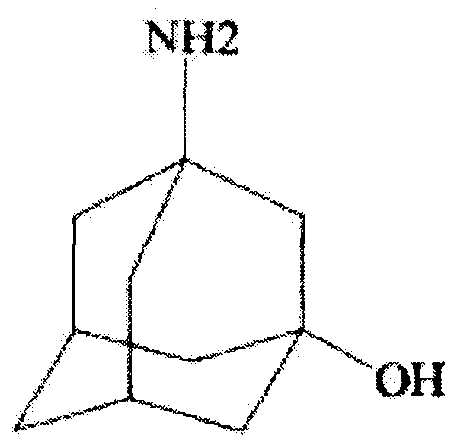
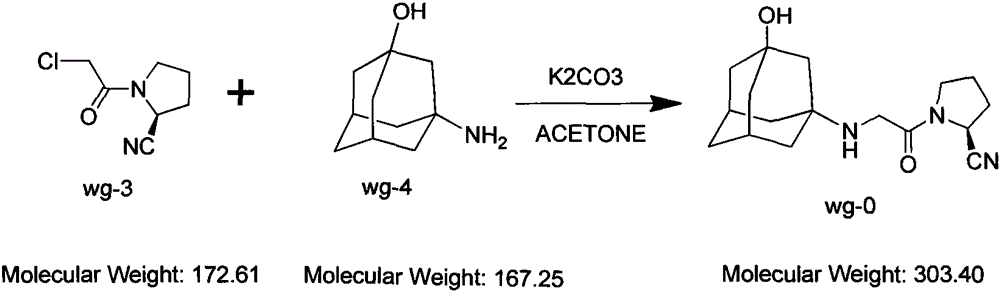
Improved industrialization technology for preparing Vildagliptin
InactiveCN105153004ABroad developmentThe value of a wide range of applicationsOrganic chemistryFiltrationDouble salt
The invention discloses a novel preparation method of Vildagliptin. The preparation method comprises that L-prolinamide as a raw material, chloroacetyl chloride and tetrahydrofuran undergo an acylation reaction, the reaction product is subjected to suction filtration, the filtrate and trifluoroacetic anhydride directly undergo a dehydration reaction without filtrate separation purification to produce (-)-(2S)-1-chloroacetylpyrrolidine-2-carbonitrile (II), the refined (-)-(2S)-1-chloroacetylpyrrolidine-2-carbonitrile, 3-amino-1-adamantanol (III), potassium carbonate and potassium iodide undergo a reaction to produce (-)-(2S)-1-[[(3-hydroxytricyclo[3.3.1.1[3,7]]dec-1-yl)amino]acetyl]pyrrolidine-2-carbonitrile (I) in acetone, and the (-)-(2S)-1-[[(3-hydroxytricyclo[3.3.1.1[3,7]]dec-1-yl)amino]acetyl]pyrrolidine-2-carbonitrile (I) is refined by calcium double salt, ethyl acetate and butanone to form pure Vildagliptin (compound wg-0). The improved synthesis technology has the advantages of less reaction steps, operation simpleness, after-treatment simpleness, low employee cost, equipment cost and time cost, high yield, high product quality and industrialization feasibility.
Owner:BEIJING KCODE PHARMA R &D CO LTD
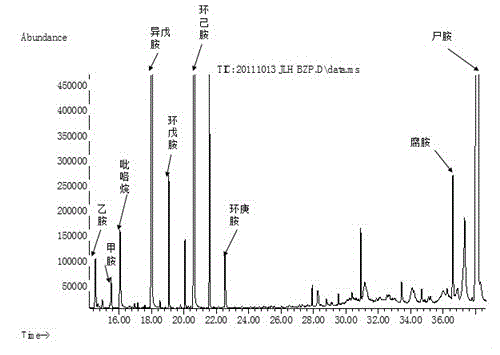
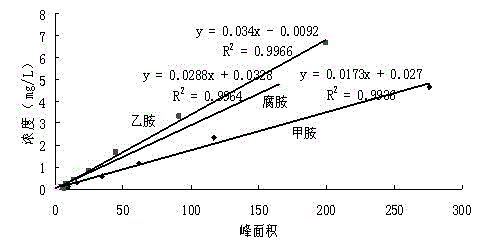
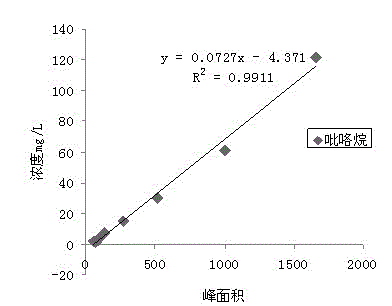
Qualitative and quantitative method for various biogenic amines in white wine
ActiveCN102914606AHigh feasibilityHigh precisionComponent separationDansyl chlorideRelative standard deviation
The invention discloses a qualitative and quantitative method for various biogenic amines in white wine and belongs to the technical field of white wine analysis and detection. According to the qualitative and quantitative method, the liquid-liquid extraction is utilized to enrich the biogenic amines in the white wine; derivation is required by both gas phase qualitative treatment and liquid phase quantitative treatment; a derivation agent adopted by the gas phase is TFAA (Trifluoroacetic Anhydride); and dansyl chloride precolumn derivatization is taken as the derivation agent for the liquid phase. According to the method provided by the invention, 9 biogenic amines are firstly determined in the white wine; the limit of detection of the quantitative method can be reduced to about 10ug / L; the linearly dependent coefficient is more than 0.99; the recovery rate is 80.19%-104.05%; the relative standard deviation is within 4%; the biogenic amines in the white wine can be detected by using the qualitative and quantitative method; the feasibility is excellent; and the precision is high.
Owner:JIANGNAN UNIV
Method for synthesizing trifluoromethyl-1,2,4-triazinone compound through copper catalyzed synthesis
InactiveCN107118168AImprove adaptabilityHigh yieldOrganic chemistrySynthesis methodsRoom temperature
The invention discloses a method for synthesizing a trifluoromethyl-1,2,4-triazinone compound through copper catalyzed synthesis. The method comprises the following steps: by taking copper salt as a catalyst, and nitrine, alkyne and trifluoroacetic anhydride as raw materials, adding triethylanmine, stirring for 1-16 hours at room temperature in a tetrahydrofuran solvent, and after the reaction is completed, treating a reaction liquid, thereby obtaining the trifluoromethyl-1,2,4-triazinone compound. The synthesis method disclosed by the invention has the advantages of being cheap in catalyst price, low in toxicity, gentle in reaction condition, high in yield, easy in raw material obtaining, simple and convenient to operate, high in functional group universality and the like, and has good industrial application prospects.
Owner:FUZHOU UNIV
Preparation method of 17 alpha-hydroxyl steroid ester
ActiveCN103665078AImprove protectionMild reaction conditionsSteroids preparationCyclohexanoneOrthoester
The invention discloses a preparation method of a steroid, and particularly relates to a preparation method of 17 alpha-hydroxyl steroid ester. 17 alpha, 21-dyhydroxyl steroid reacts with orthoester to form a steroid intermediate containing 1, 3-dioxane-5-cyclohexanone, and then 17 alpha-hydroxyl steroid ester is obtained through hydrolysis. Compared with the prior art, the preparation method has the advantages that highly amyctic trifluoroacetic anhydride is avoided, the reaction condition is mild, a solid acid can be adopted for catalytic hydrolysis, the catalytic acid is recycled, so that environmental protection is facilitated, and the method has low pollution, simple technology and mild reaction condition, and is suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
High k dielectric growth on metal triflate or trifluoroacetate terminated iii-v semiconductor surfaces
InactiveUS20080241423A1Polycrystalline material growthSemiconductor/solid-state device manufacturingTrifluoromethanesulfonic anhydrideTrifluoroacetic acid
Surface preparation of a compound semiconductor surface, such as indium antimonide (InSb), with a triflating agent, such as triflic anhydride or a trifluoroacetylating agent, such as trifluoroacetic anhydride is described. In one embodiment, the triflating or trifluoroacetylating passivates the compound semiconductor surface by terminating the surface with triflate trifluoroacetate groups. In a further embodiment, a triflating agent or trifluoroacetylating agent is employed to first convert a thin native oxide present on a compound semiconductor surface to a soluble species. In another embodiment, the passivated compound semiconductor surface is activated in an ALD chamber by reacting the triflate or trifluoroacetate protecting groups with a protic source, such as water (H2O). Metalorganic precursors are then introduced in the ALD chamber to form a good quality interfacial layer, such as aluminum oxide (Al2O3), on the compound semiconductor surface.
Owner:INTEL CORP
N-full aromatic hydrocarbyl bisphenol-diamine tetrafunctional fluorene-based benzoxazine and preparation method thereof
InactiveCN103936765AIncrease crosslink densityImprove toughnessOrganic chemistryMannich condensationParaformaldehyde
The invention provides N-full aromatic hydrocarbyl bisphenol-diamine tetrafunctional fluorene-based benzoxazine and a preparation method thereof. A new N-full aromatic hydrocarbyl bisphenol-diamine tetrafunctional benzoxazine monomer is obtained by the following steps: using trifluoroacetic anhydride to protect amino in 2, 7-dihydroxy-9, 9-bis-(4-amino phenyl) fluorene to produce 2, 7-dihydroxy-9, 9-bis-(4-trifluoroacetyl phenyl amino) fluorene, then performing Mannich condensation reaction of the 2, 7-dihydroxy-9, 9-bis-(4-trifluoroacetyl phenyl amino) fluorene and aliphatic amine and paraformaldehyde to produce a 9, 9-bis-(4-trifluoroacetyl phenyl amino) bisphenol fluorene-based benzoxazine monomer, then performing amino deprotection, and performing secondary Mannich condensation reaction with a phenolic compound and paraformaldehyde to finally obtain the new N-full aromatic hydrocarbyl bisphenol-diamine tetrafunctional benzoxazine monomer. The problems that fluorene-based benzoxazine with a structure with larger steric hindrance is small in molecular weight, low in crosslinking density, and poor in toughness and thermal performance reduction caused by the introduction of flexible groups can be solved, and the processing properties of polymers can be improved.
Owner:HARBIN ENG UNIV
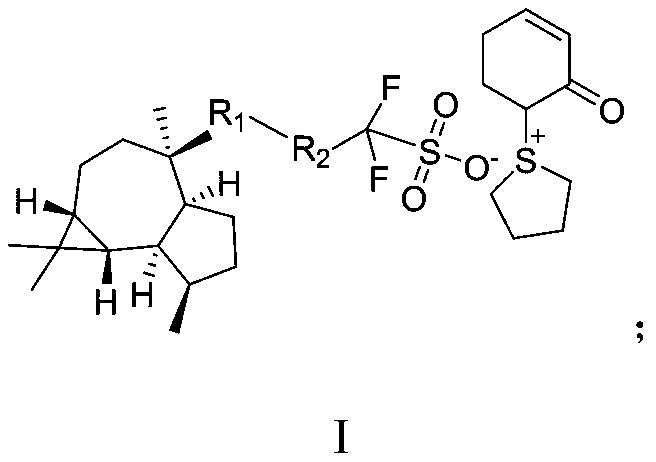
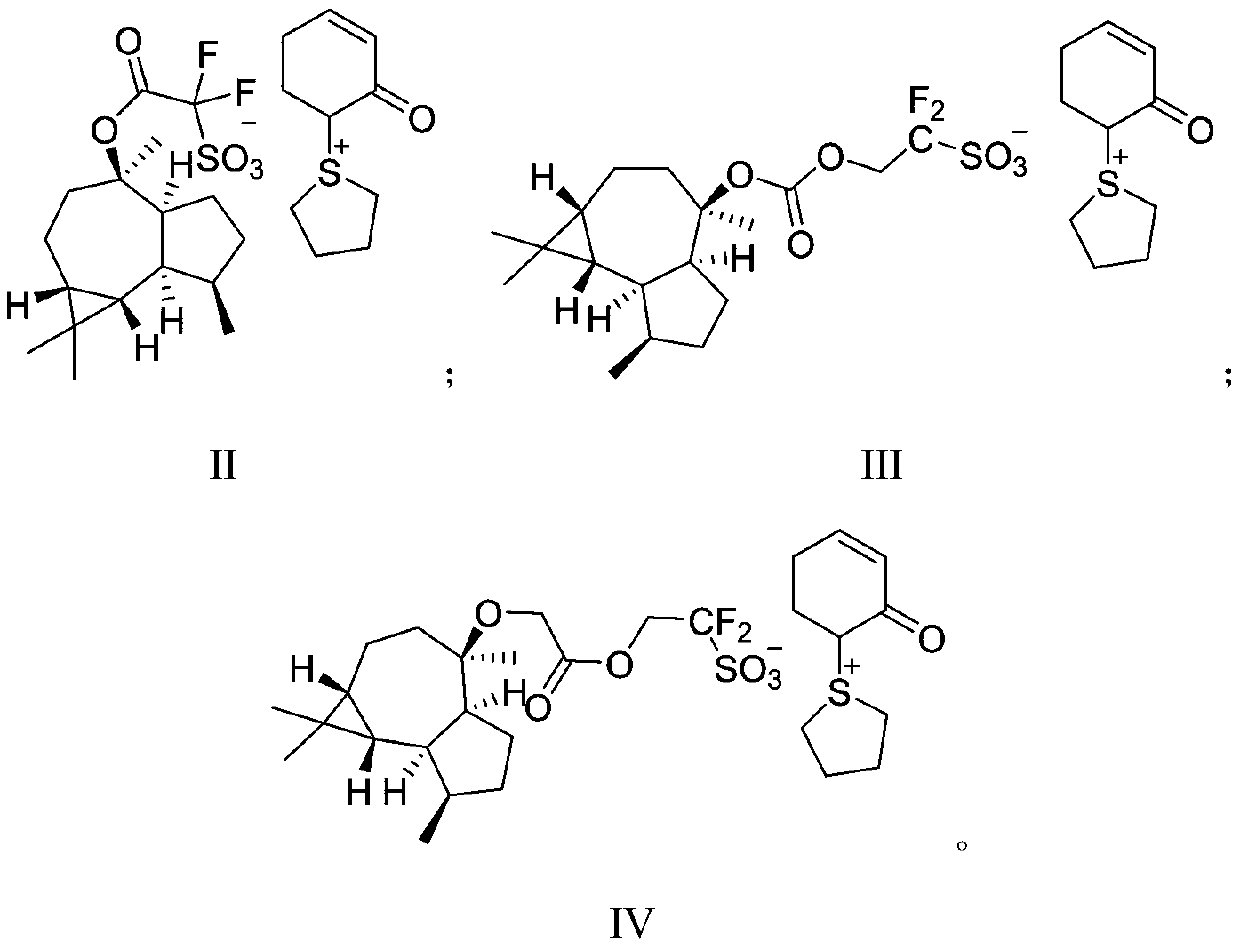
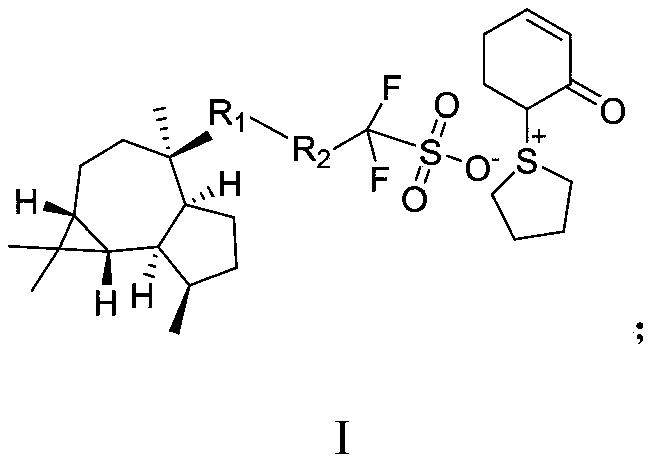
Sulfonium sulfonate salt photoacid generator synthesized from ledol and synthesis method for sulfonium sulfonate salt photoacid generator
PendingCN111138407AReduce spreadHigh molecular weightOrganic chemistryPhotosensitive materials for photomechanical apparatusChemical synthesisSilanes
The invention discloses a sulfonium sulfonate salt photoacid generator synthesized from ledol and a synthesis method for the sulfonium sulfonate salt photoacid generator, belonging to the fields of chemical synthesis and photoetching materials. The photoacid generator has a structural general formula which is described in the specification. In the structural general formula, R1 is one selected from the group consisting of groups which are described in the specification; and R2 is one selected from the group consisting of a covalent bond, an alkyl group, a cycloalkyl group, an ester group-containing alkyl group and a fluorine-containing alkyl group. The synthesis method for the photoacid generator comprises the following steps: allowing the ledol to react with a sulfonate compound so as toobtain an intermediate; and allowing the intermediate to react with (cyclohexyl-1,5-dienyloxy)-trimethyl-silane, tetramethylene sulfoxide and trifluoroacetic anhydride so as to obtain the sulfonium sulfonate salt photoacid generator. According to the invention, a raw material, namely the ledol adopted in the method provided by the invention has large molecular weight, so the photoacid generator formed by the ledol also has large molecular weight; diffusion of the photoacid generator can be reduced; and improvement of the edge roughness, reduction of the line width roughness and improvement ofthe resolution ratio are facilitated.
Owner:上海博栋化学科技有限公司
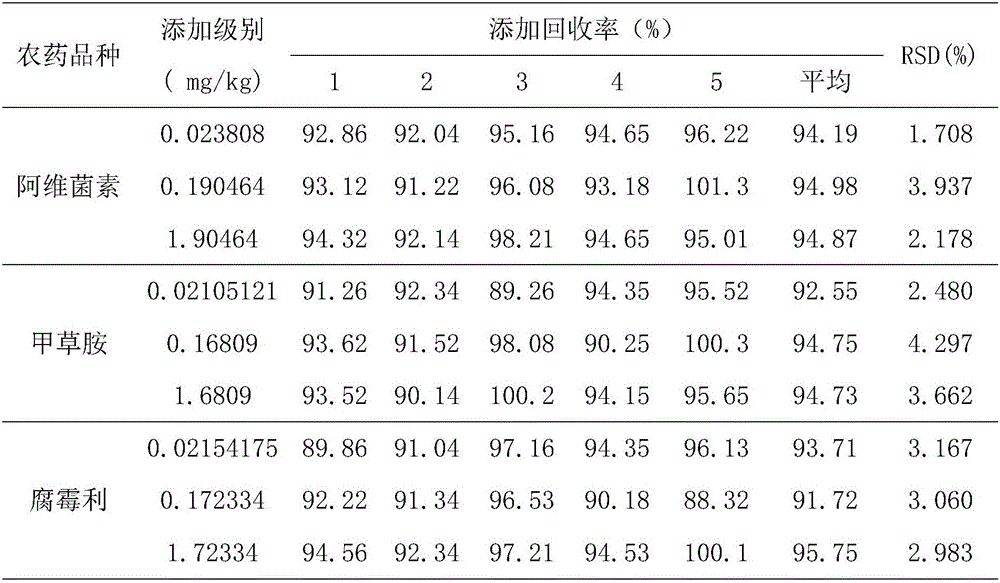
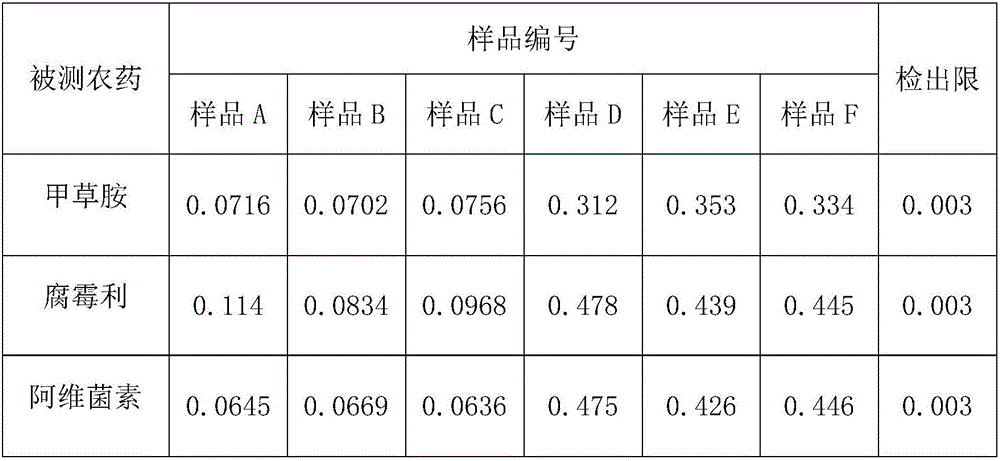
High performance liquid chromatography detection method for abamectin content in edible vegetable oil
InactiveCN106226444AEfficient extractionLow costComponent separationLiquid Chromatography-FluorescencePretreatment method
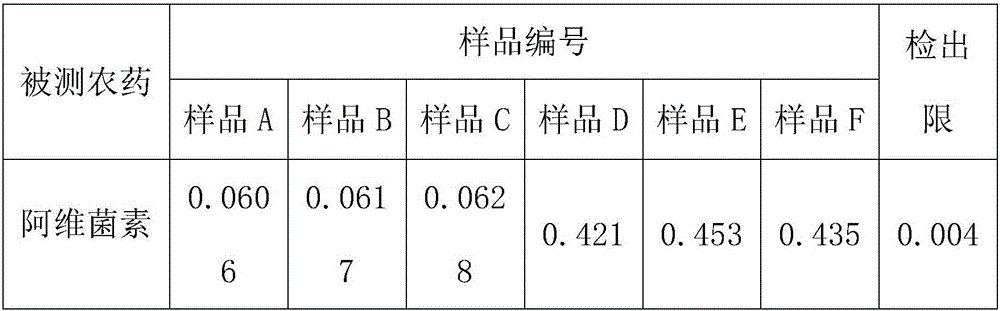
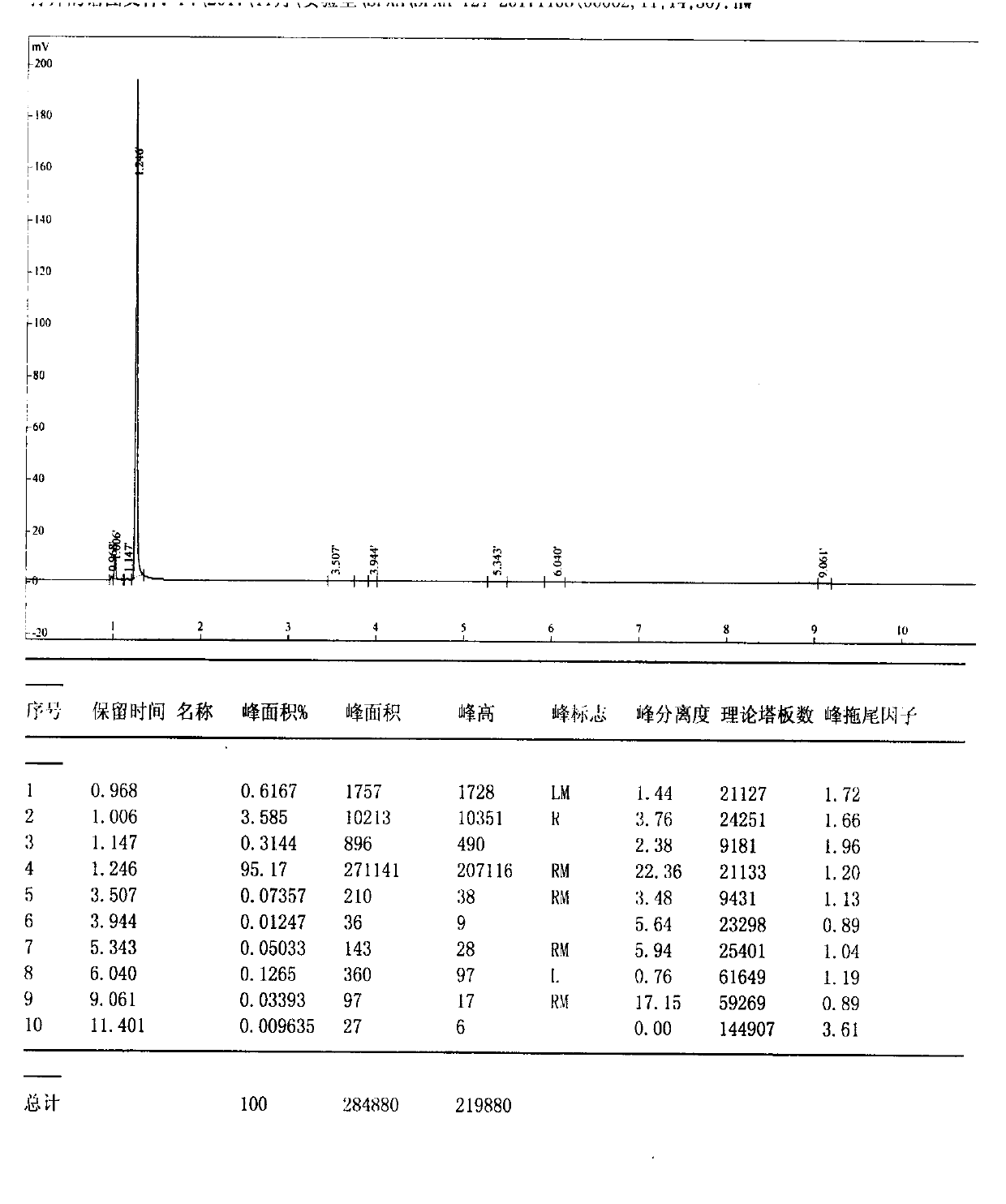
The invention discloses a high performance liquid chromatography detection method for the abamectin content in edible vegetable oil. The method comprises the steps that a sample is subjected to vortex oscillation extraction through methanol, purified with an ODS C18 solid-phase extraction column, subjected to derivatization through N-methylimidazole and trifluoroacetic anhydride and lastly determined through a liquid chromatography-fluorescence method. According to the method, few reagents are adopted, and the cost is reduced; a sample pretreatment method is simple, the purification effect is good, maintenance of instruments in the using process is reduced, and the technical problems that when a liquid chromatography-ultraviolet detector is used, the sensitivity is too low, and matrix interference is serious are solved; a determined result is accurate and good in repeatability, the detection specificity and sensitivity are high, and a reference is provided for detection of the abamectin content in the edible vegetable oil.
Owner:FOSHAN HAIYUE ZHIDA TECH CO LTD
Clean and high-conversion-rate preparing method of 2,2,2-trifluroacetophenone
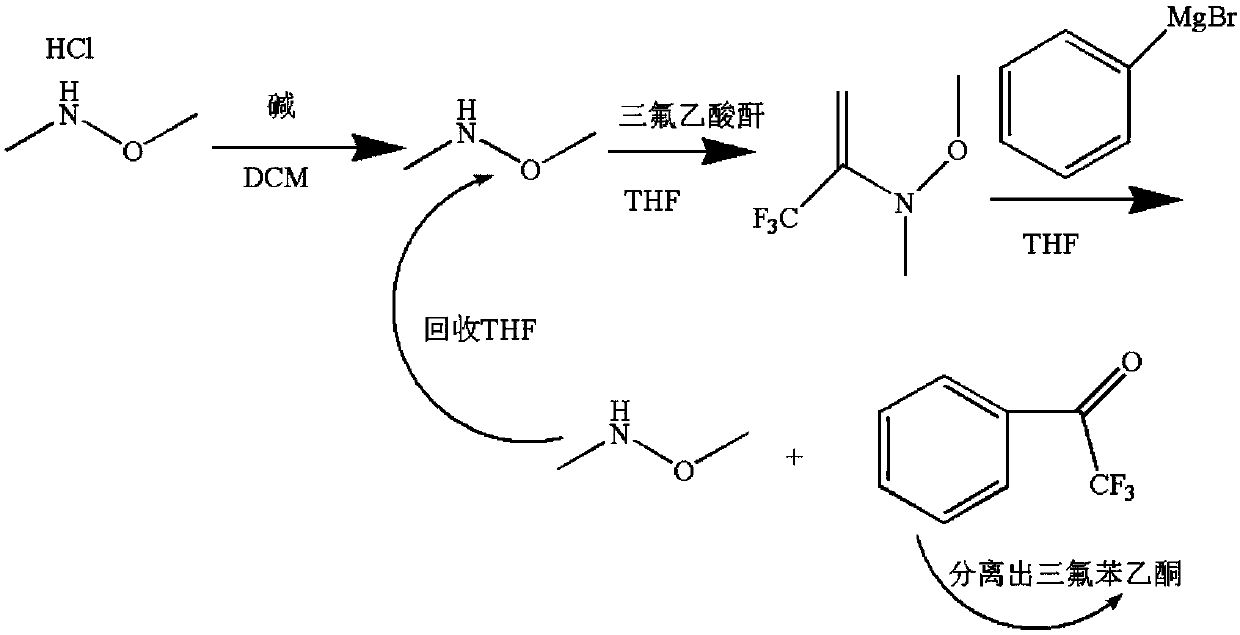
InactiveCN108047019AImprove conversion rateEfficient preparationPreparation by organometalhalide reactionOrganic compound preparationGrignard reagentSolvent
The invention discloses a clean and high-conversion-rate preparing method of 2,2,2-trifluroacetophenone. The method includes the following steps of S1, making N,O-dimethylhydroxylamine hydrochloride as the raw material react with organic alkali to obtain free N,O-dimethylhydroxylamine; S2, making trifluoroacetic anhydride react with free N,O-dimethylhydroxylamine to generate N,O-dimethyltrifluoroacetamide; S3, making a Grignard reagent react with N,O-dimethyltrifluoroacetamide to prepare trifluroacetophenone, recovering N,O-dimethylhydroxylamine generated in the step S1, applying N,O-dimethylhydroxylamine to the step S2, and recycling the reaction solvents used in the steps S1, S2 and S3. By means of the preparing method, prepared high-nucleophilicity N,O-dimethyltrifluoroacetamide reactswith the Grignard reagent, 2,2,2-trifluroacetophenone is efficiently prepared, meanwhile produced byproducts can be applied for preparing N,O-dimethyltrifluoroacetamide, and a recycling effect is achieved.
Owner:GUANGXI WANDE PHARMA
Azo-furazan compound and preparing method thereof
The invention discloses an azo-furazan compound and a preparing method thereof. The preparing method includes the specific steps that malononitrile serves as a raw material and is reacted with an oxidizing agent to obtain an amino-oximido furazan intermediate; the amino-oximido furazan intermediate is reacted with different cyclization reagents (triethyl orthoformate, bromized nitrile, acetic anhydride, trifluoroacetic anhydride and the like) to obtain an amidogen-substituted furazan intermediate, then the furazan intermediate is reacted with potassium permanganate and hydrochloric acid of 10%-20%, and the azo-furazan compound is separated and precipitated. The preparing method is simple; compared with the prior art, azo-furazan compounds with different substituent groups can be prepared at a time in a high throughput mode, operation is safe, and cost is low.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
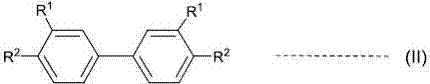
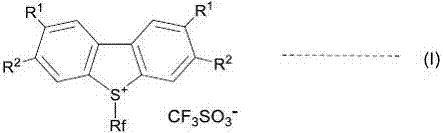
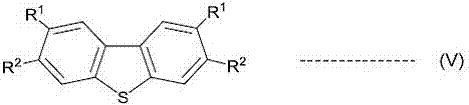
A novel method for preparing S-(perfluoroalkyl)-dibenzothiophenium trifluoromethanesulfonate
ActiveCN107540655ALow costOrganic compound preparationSulfonic acids salts preparationSulfonateTrifluoroacetic acid
A method for preparing S-(perfluoroalkyl)-dibenzothiophenium trifluoromethanesulfonate shown as a general formula (I) by a one-pot process with significant effects is disclosed by the invention. The method includes reacting a biphenyl compound, perfluoroalkyl sulfonate, trifluoroacetic anhydride and trifluoromethanesulfonic acid. The biphenyl compound can be recovered and utilized so that the method is an environmentally friendly preparing method.
Owner:浙江瑞博制药有限公司
Gas chromatographic column pretreatment method for high-viscosity alcohol or/and amines
The invention discloses a gas chromatographic column pretreatment method for high-viscosity alcohol or / and amines. The pretreatment method comprises the following steps of: placing 0.5-1mL of derivatization reagent trifluoroacetic anhydride in a volumetric flask with the volume of 5mL; adding 10-50mg of high-viscosity alcohol or / and amines in the volumetric flask at the temperature of 0-5 DEG C; oscillating the mixture for 0.5-1 minute; reacting for 10-30 minutes at the temperature of 30-60 DEG C; and cooling to the temperature of 20-25 DEG C to obtain a sample for gas chromatography detection. The pretreatment method is mainly used for quickly analyzing high-viscosity alcohol or / and amines.
Owner:XIAN MODERN CHEM RES INST
Method for quantitating abamectin, alachlor and procymidone in edible vegetable oil
The invention discloses a method for quantitating abamectin, alachlor and procymidone in edible vegetable oil. A sample is subjected to methanol vortex oscillation extraction and purified with an Envi-18 solid-phase extraction column, the content of alachlor and procymidone in part of the sample is directly analyzed with a gas chromatograph, the rest sample is subjected to derivatization with N-methylimidazole and trifluoroacetic anhydride, and the content of abamectin is determined with a liquid chromatogram-fluorescence method. Fewer reagents are adopted in the method, abamectin, alachlor and procymidone in the edible vegetable oil can be effectively extracted, and the cost is saved; the sample pretreatment method is simple, a cleaner loading solution is provided, and maintenance of instruments during usage is reduced; measured results are accurate, the repeatability is good, detection specificity and sensitivity are high, and the reference is provided for simultaneous detection of the content of abamectin, alachlor and procymidone in the edible vegetable oil.
Owner:厦门泓益检测有限公司 +3
Synthetic method of efavirenz key intermediate
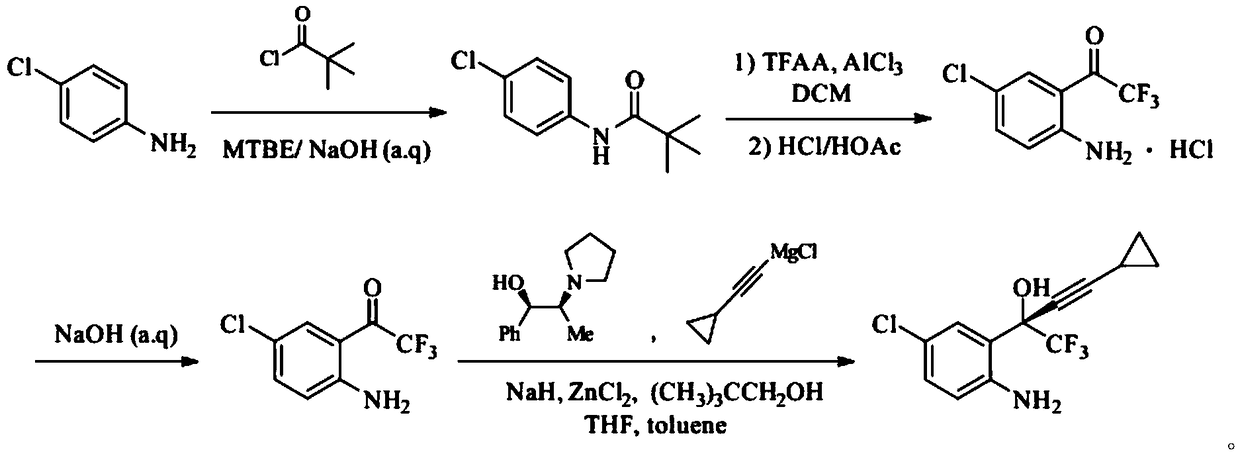
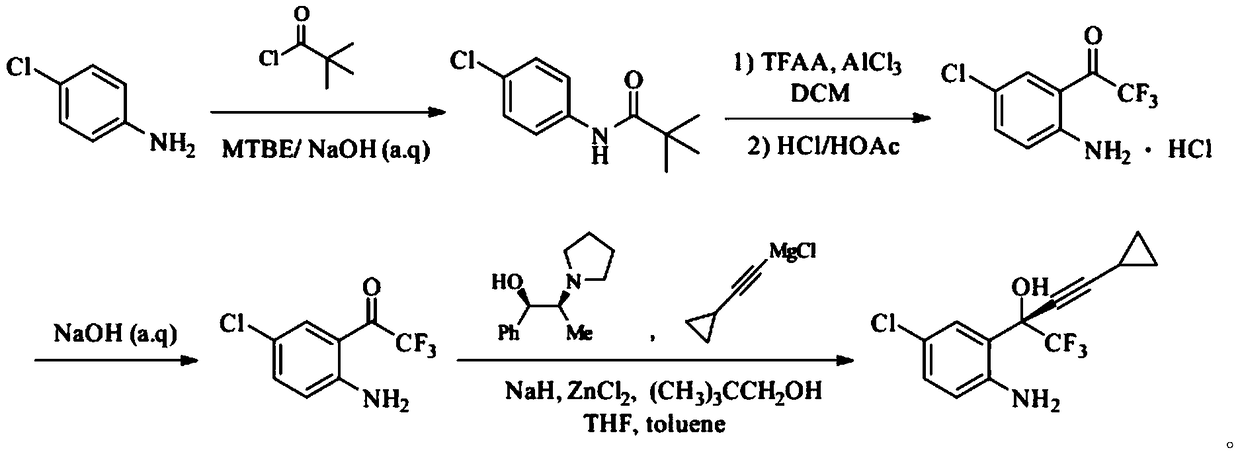
ActiveCN108947855ALow toxicityRaw materials are cheap and easy to getOrganic compound preparationOrganic chemistry methodsAnilineAniline hydrochloride
The invention provides a synthetic method of an efavirenz key intermediate. The synthetic method comprises the following steps: carrying out reaction on parachloroaniline and pivaloyl chloride to protect amino to obtain N-(4-chlorphenyl)-2,2-dimethyl propanamide; carrying out Friedel-Crafts acylation reaction on the product and Friedel-Crafts acylation under action of aluminum trichloride to hydrolyze to obtain 4-chloro-2-trifluoroacetyl aniline hydrochloride in an acidic condition; and then carrying out alkalization to obtain 4-chloro-2-trifluoroacetyl aniline, carrying out reaction with cyclopropyl acetylene magnesium chloride in a catalytic system formed by a ligand (1R, 2S)-1-phenyl-2-(1-pyrrolidyl)-1-propyl alcohol, and carrying out an asymmetrical self-catalytic reaction to obtain the efavirenz key intermediate. The synthetic method of the efavirenz key intermediate, provided by the invention, is cheap and easily available in raw material, low in toxicity of reagent and mild in reaction condition, amino protection and deprotection are not carried out frequently, the line is concise, the yields of reaction of each step are excellent, and the total yield is high.
Owner:JIANGSU SHAXING CHEM
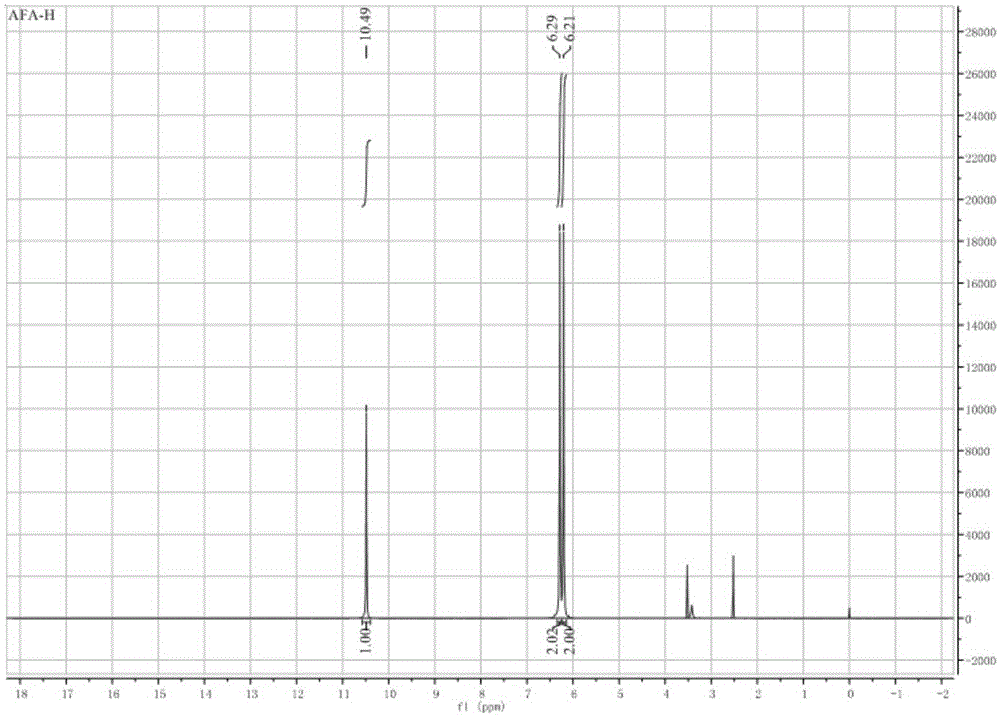
Synthetic method of 2-hydroxy propanedinitrile
InactiveCN102936209ASynthetic cleanSafe and efficient synthesisCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisAcetic anhydride
The invention relates to a practical synthetic method of 2-hydroxy propanedinitrile based on a chemical synthesis method, mainly solving the problems of conventional synthetic method that the specific operation is complex, the reaction condition is severe, the reaction time is long, the performability is low, and the industrial production cannot be carried out. According to the synthetic method, the conventional 2-(1-hydroxy acetal) propanedinitrile which is easy to obtain is adopted and reacts with peroxy acid to generate the 2-hydroxy propanedinitrile, wherein the peroxy acid is prepared by acetic anhydride or trifluoroacetic anhydride and urea hydrogen peroxide. The chemical synthetic method of 2-hydroxy propanedinitrile provided by the invention is brand new, short in reaction time, low in cost, and suitable for industrial scale production.
Owner:SHANGHAI STA PHARMA R&D CO LTD
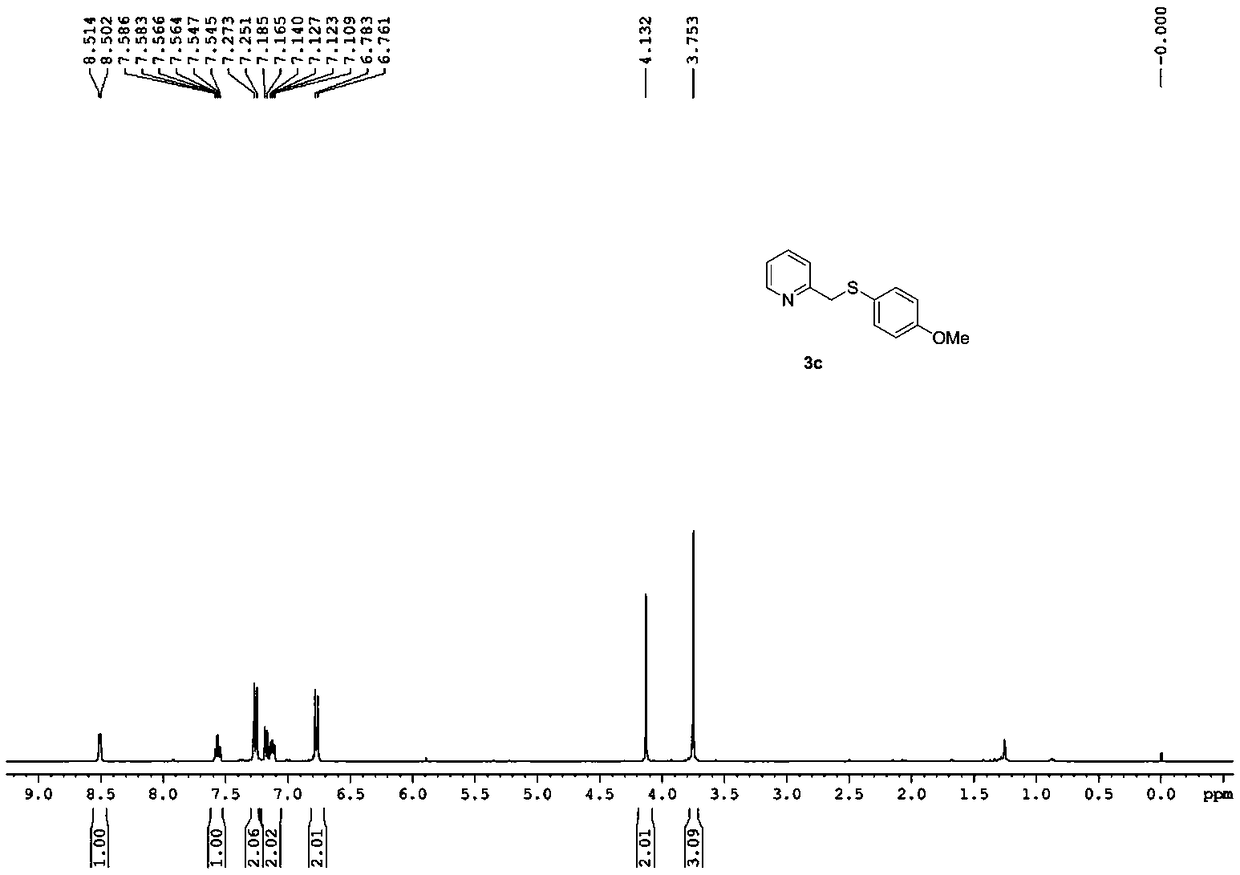
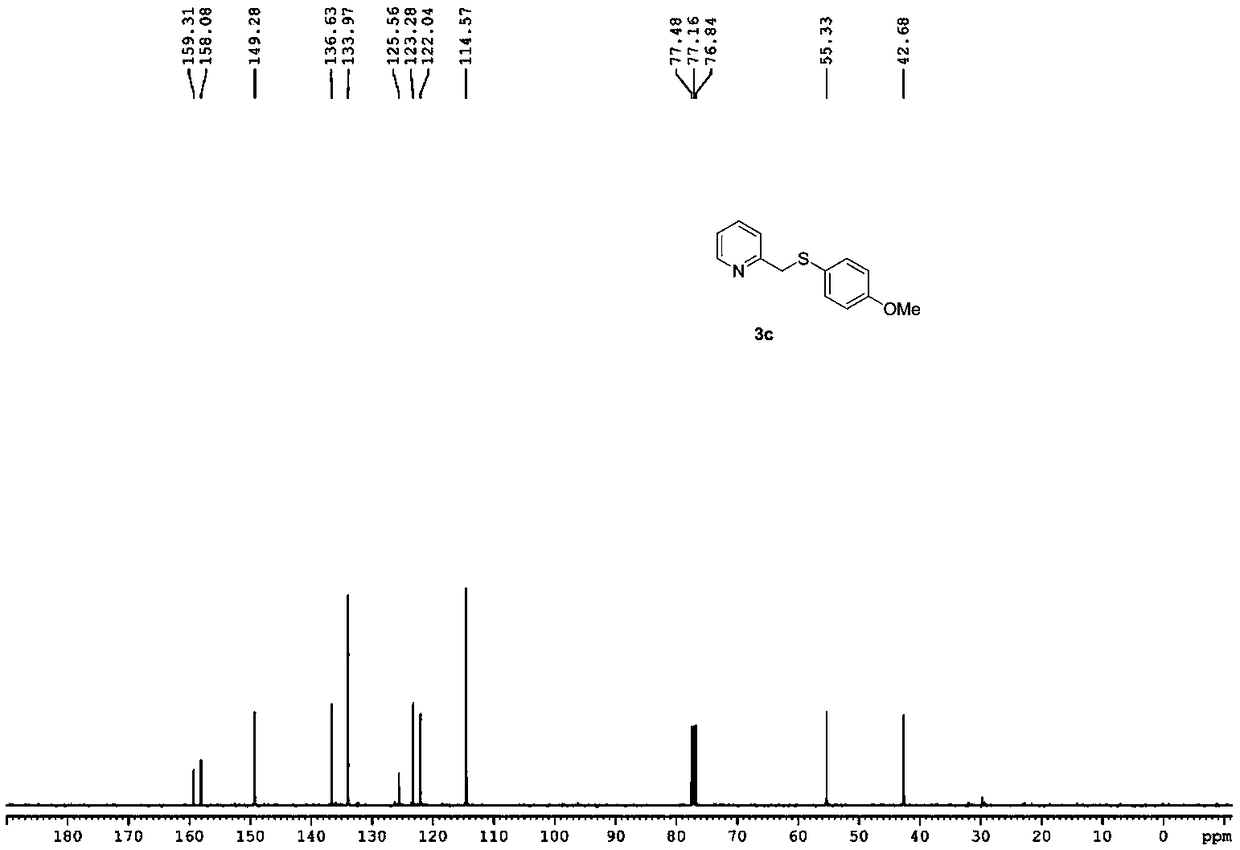
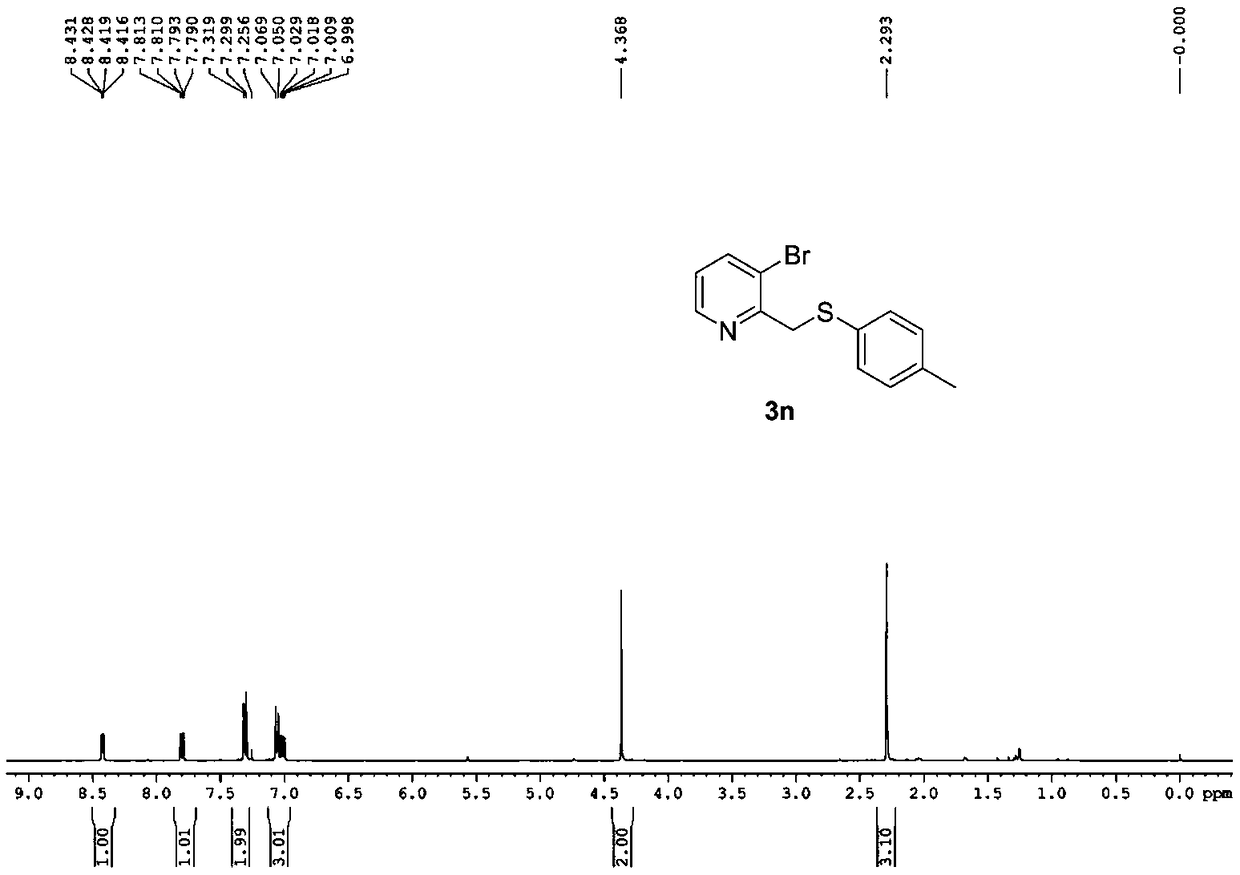
Synthesizing method of 2-pyridine methyl sulfide and synthesizing process of related drugs
ActiveCN109134354AStrong position selectivityHigh yieldOrganic chemistryLithium bromidePyridine-N-oxide
The invention relates to a simple synthesizing method of 2-pyridine methyl sulfide and related drugs. The method is characterized in that 2-methyl pyridine n-oxide is used as the raw material and pyridine n-oxide or dichloromethane is used as the solvent to have reaction with trifluoroacetic anhydride to obtain a trifluoroacetate intermediate, purification is not needed, the trifluoroacetate intermediate is allowed to have reaction with thiophenol under the catalysis of lithium bromide or tetrabutyl ammonium bromide and by using toluene or ethyl acetate as the solvent to generate the 2-pyridine methyl sulfide. The method is simple to operate, cheap in reagents, easy in reagent obtaining, mild in reaction conditions, wide in substrate applicability, good in position selectivity, high in yield and the like. In addition, the method is successfully applied to the synthesizing of omeprazole sulfide and rabeprazole sulfide, and the synthesizing method does not need catalysts.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
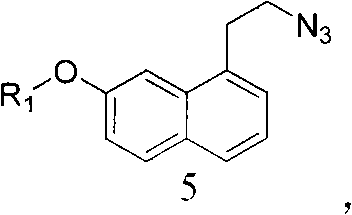
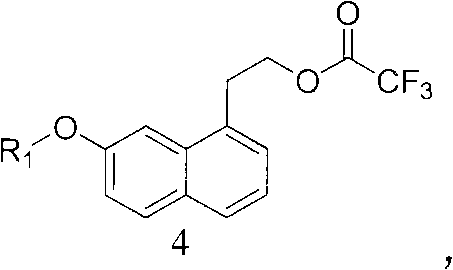
Intermediate for preparing agomelatine and relevant preparation method
ActiveCN102531956AHigh purityHigh yieldOrganic compound preparationCarboxylic acid esters preparationCarboxylic acidSodium azide
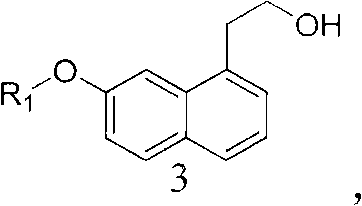
The invention relates to the technical field of preparation methods of carboxylic acid amide, in particular to the technical field of preparation methods of agomelatine and an intermediate thereof. The invention particularly relates to an intermediate of agomelatine and a relevant preparation method. The preparation method comprises the following steps of: undergoing an alkylation reaction on 1-halogen-7-naphthalene alkoxyl serving as a raw material to obtain a compound shown as a formula (2); undergoing a Grignard reaction on the obtained compound shown as the formula (2) to obtain a compound shown as a formula (3); reacting the obtained compound shown as the formula (3) with trifluoroacetic anhydride to obtain a compound shown as a formula (4), wherein the compound shown as the formula (4) is a novel intermediate compound for synthesizing agomelatine and a derivative thereof; reacting the compound shown as the formula (4) with sodium azide simultaneously to obtain a compound shown as a formula (5), wherein the compound shown as the formula (5) is a novel intermediate compound for synthesizing agomelatine and a derivative thereof; and undergoing simple type reactions such as hydrogenation reduction, electrophilic substitution and the like on the compound shown as the formula (5) to obtain agomelatine suitable for process production and a derivative thereof, wherein R1 is alkyl; and X is F, Cl, Br or I.
Owner:浙江瑞博制药有限公司
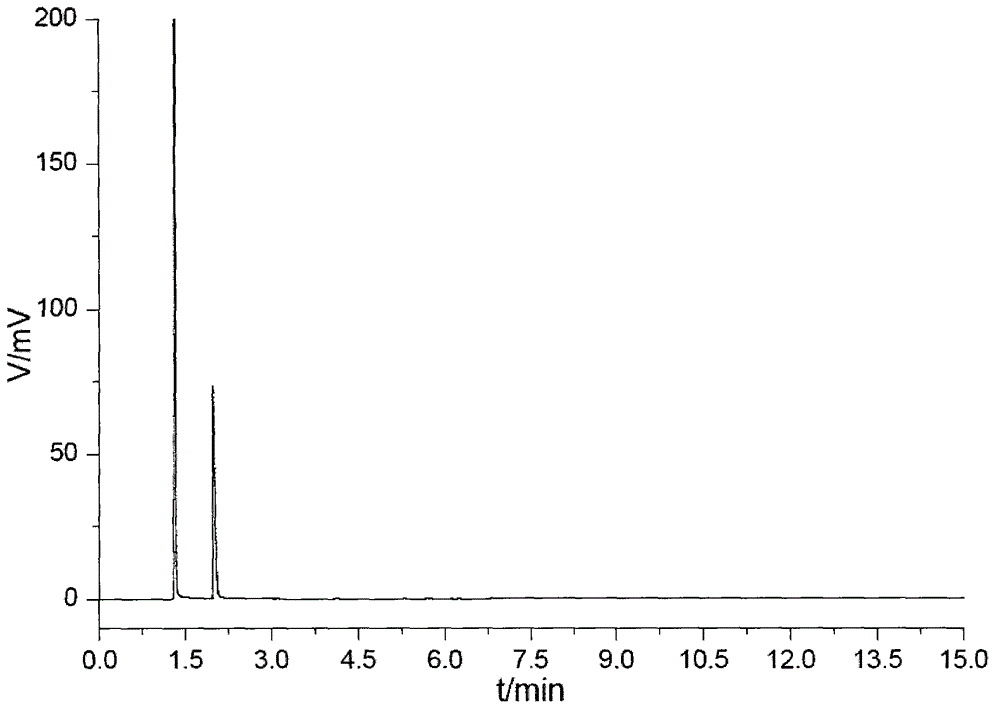
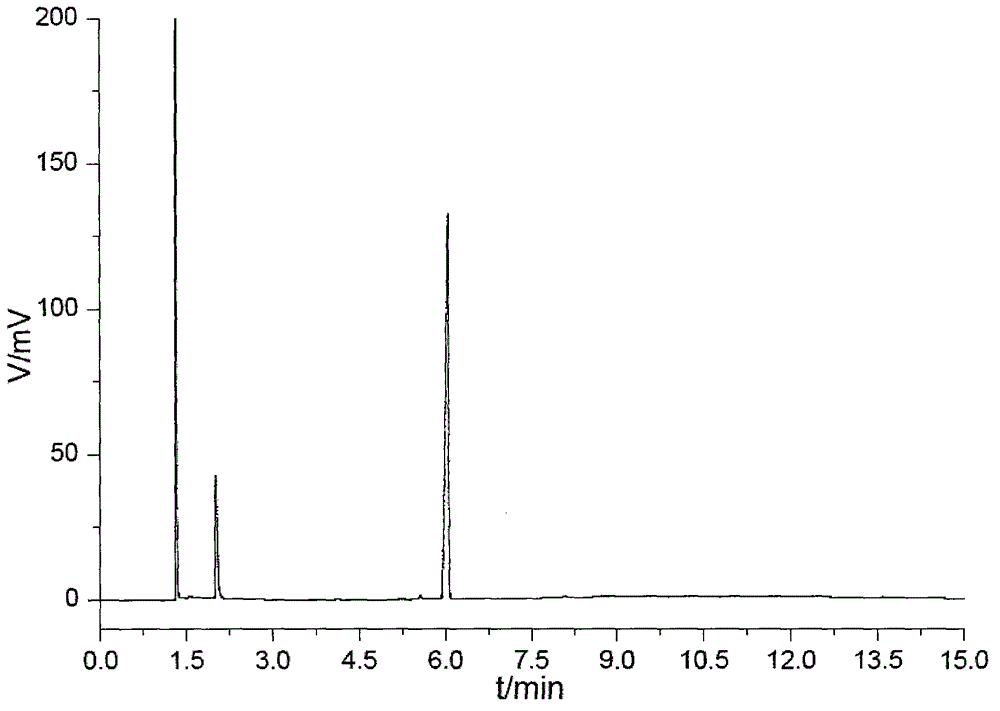
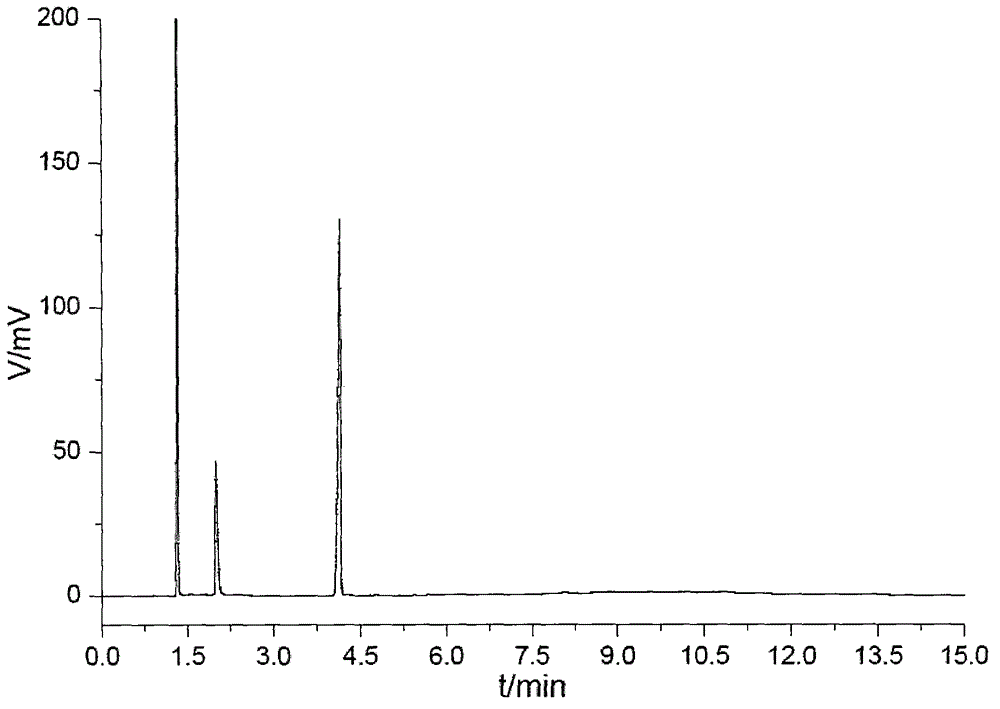
Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method
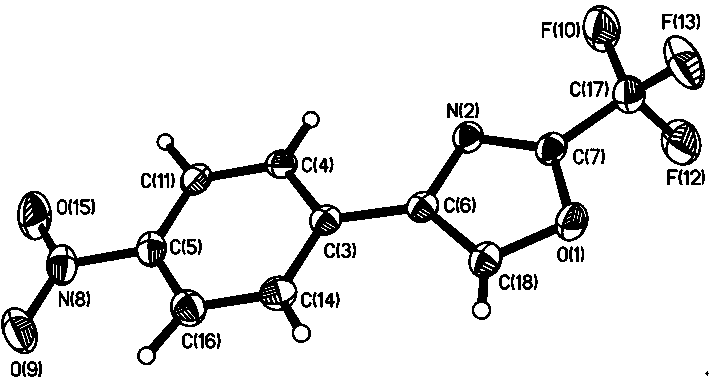
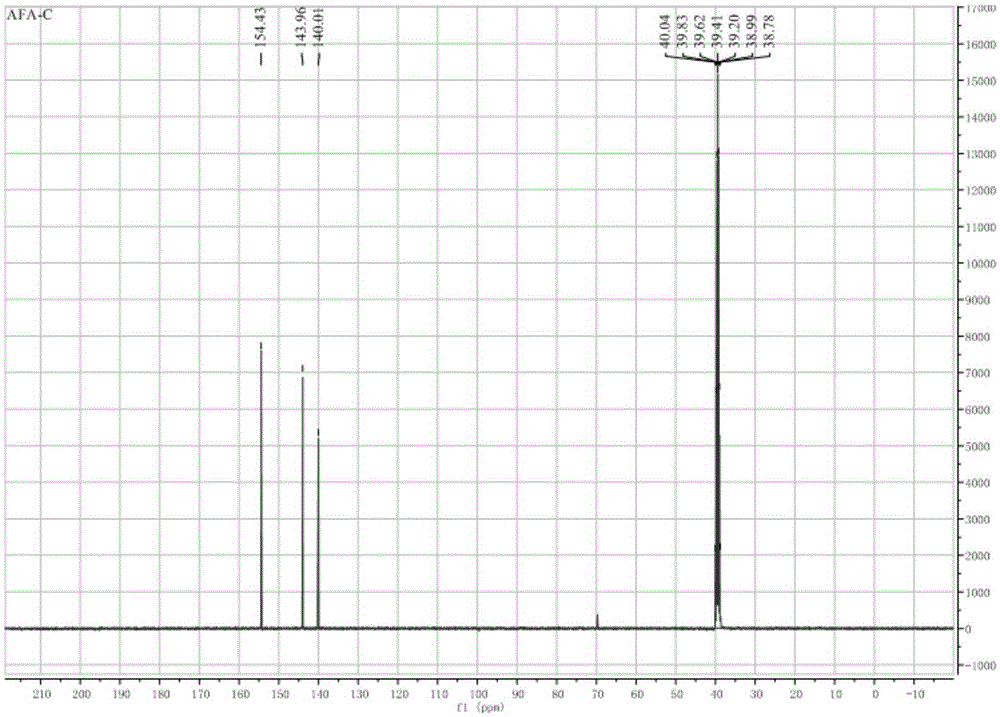
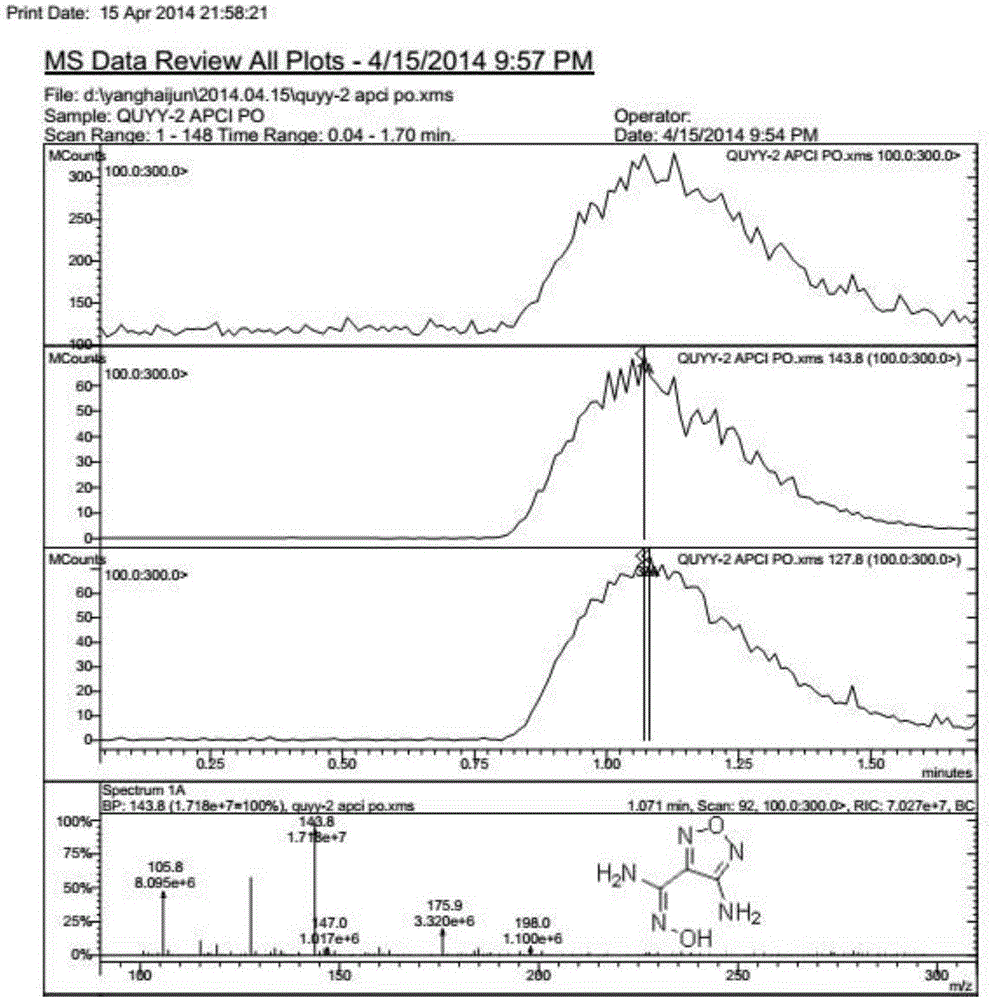
InactiveCN107163043AMild reaction conditionsHigh selectivityOrganic chemistryN dimethylformamideOrganic synthesis
The invention relates to the field of organic synthesis, and discloses a method for synthesizing a pyrazolo[1,5-a]pyridine-3-carboxylate derivative. The method comprises the following step: 1) 2-pyridine acetate or substitute 2-pyridine acetate and N,N-dimethylformamide dimethyl acetal are subjected to a reaction; 2) hydroxylamine hydrochloride is continuously added for a reaction to obtain a compound III; and 3) the compound III and trifluoroactic anhydride are subjected to the reaction to generate the product. After the reaction is completed, water is added, and the pH value is adjusted to neutrality, extraction is carried out and the organic phases are merged, and then the steps of drying, concentration and re-crystallization are carried out. The synthetic method has the advantages of easy acquisition of the raw materials, mild reaction condition and high yield; whether the raw materials have a symmetrical structure or not, the target product can be specifically obtained, post-treatment and purifying are easily operated, and amplification production can be realized.
Owner:上海毕得医药科技股份有限公司
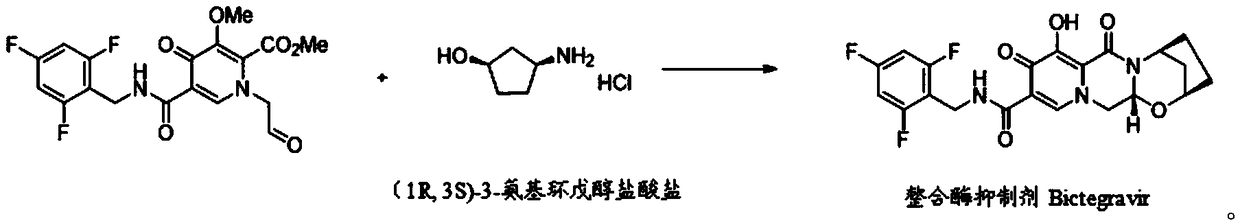
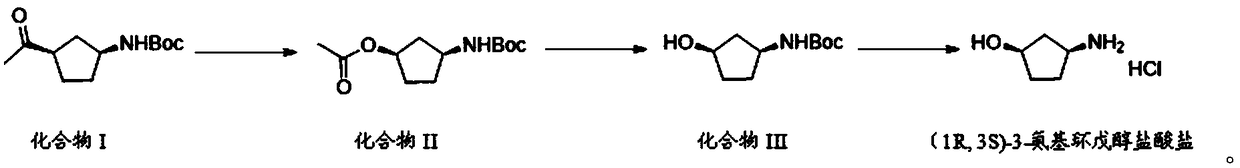
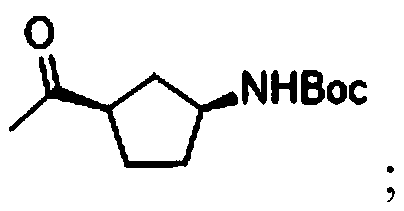
Preparation method of (1R,3S)-3-amino cyclopentanol hydrochloride
InactiveCN108774145AImprove processing stabilitySmooth precipitationCarbamic acid derivatives preparationOrganic compound preparationCarbamide peroxideCyclopentanol
The invention provides a preparation method of (1R,3S)-3-amino cyclopentanol hydrochloride. A urea peroxide-trifluoroacetic anhydride system is adopted as an oxidizing agent, and a compound I is subjected to oxidation reaction so as to generate a compound II and a compound II', so that the use of an oxidizing agent with high price and big risk is avoided. Hydrogen chloride obtained through esterification reaction of isopropanol and an acyl chloride compound is subjected to de-protection reaction with a compound III, so that the process stability is good compared with the way of directly feeding hydrogen chloride and carrying out de-protection reaction on the hydrogen chloride and the compound III, the condition that the (1R,3S)-3-amino cyclopentanol hydrochloride can be smoothly separatedout from a reaction solution is ensured, and the method is convenient to operate and friendly to the work environment. In addition, the preparation method provided by the invention is high in productyield and purity, low in production cost, high in safety, simple to operate, and suitable for large-scale production.
Owner:ANHUI TWISUN HI TECH PHARM CO LTD
Synthesis method of 3-bromo-6-chloropyridyl-2-formic acid
InactiveCN103058921ALow costSuitable for large-scale industrial productionOrganic chemistrySynthesis methodsSodium cyanide
The invention provides a synthesis method of 3-bromo-6-chloropyridyl-2-formic acid, which comprises the following steps: by using 3-bromo-6-chloropyridine as an initial raw material, carrying out oxidation reaction with urea peroxide and trifluoroacetic anhydride to obtain 3-bromo-6-chloropyridine oxynitride; reacting with trimethylsilyl cyanide and triethylamine to obtain 3-bromo-6-chloropyridyl-2-cyanide; and finally, hydrolyzing in sulfuric acid to obtain the 3-bromo-6-chloropyridyl-2-formic acid. The synthesis method provided by the invention has the advantages of high operational safety, environment friendliness and the like; and especially, the synthesis method uses low-cost low-toxicity raw materials in stead of such expensive raw material as 2-fluoro-3-bromo-6-chloropyridine and virulent raw material sodium cyanide, and is suitable for large-scale industrial production.
Owner:SUZHOU ZIKANG PHARMA INC
One-pot method used for synthesis of diaryl methyl ketal
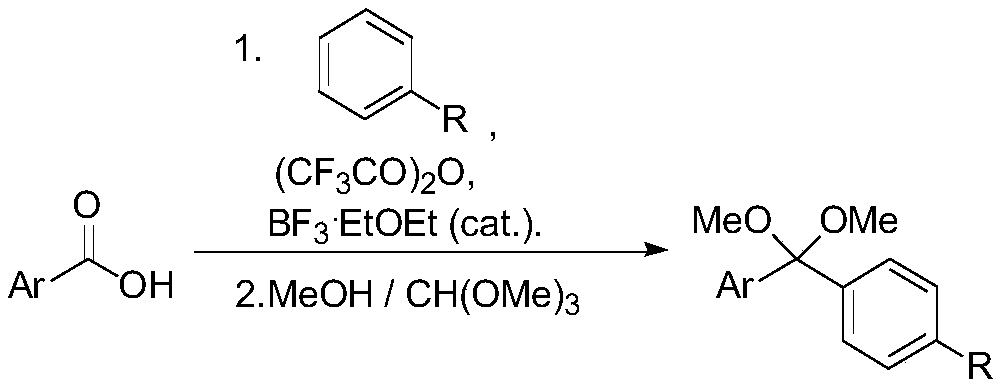
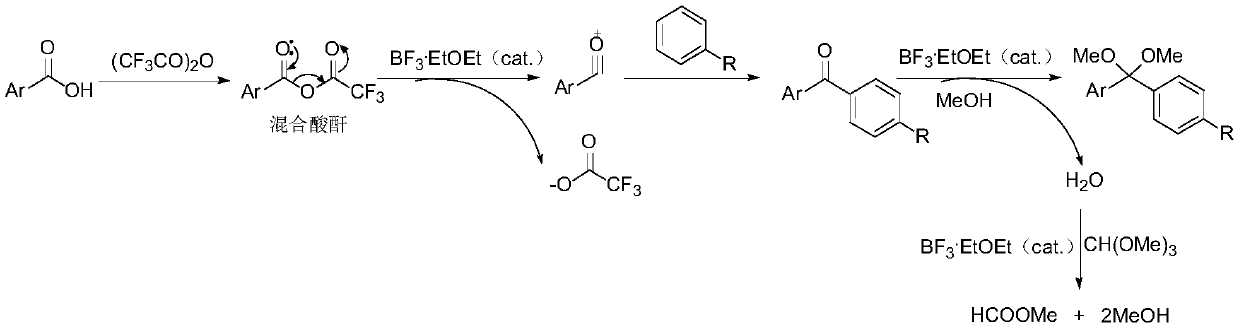
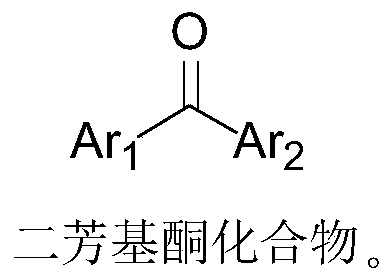
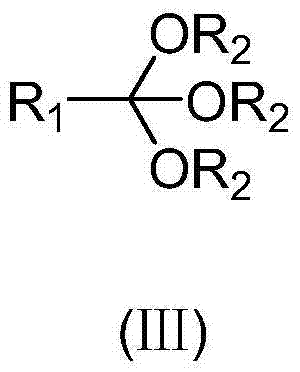
ActiveCN110396040AImplement multi-step reactionsShort stepsOrganic chemistryOrganic compound preparationBenzeneAryl
Owner:SOUTHEAST UNIV +1
Production of tertiary amines by reductive amination
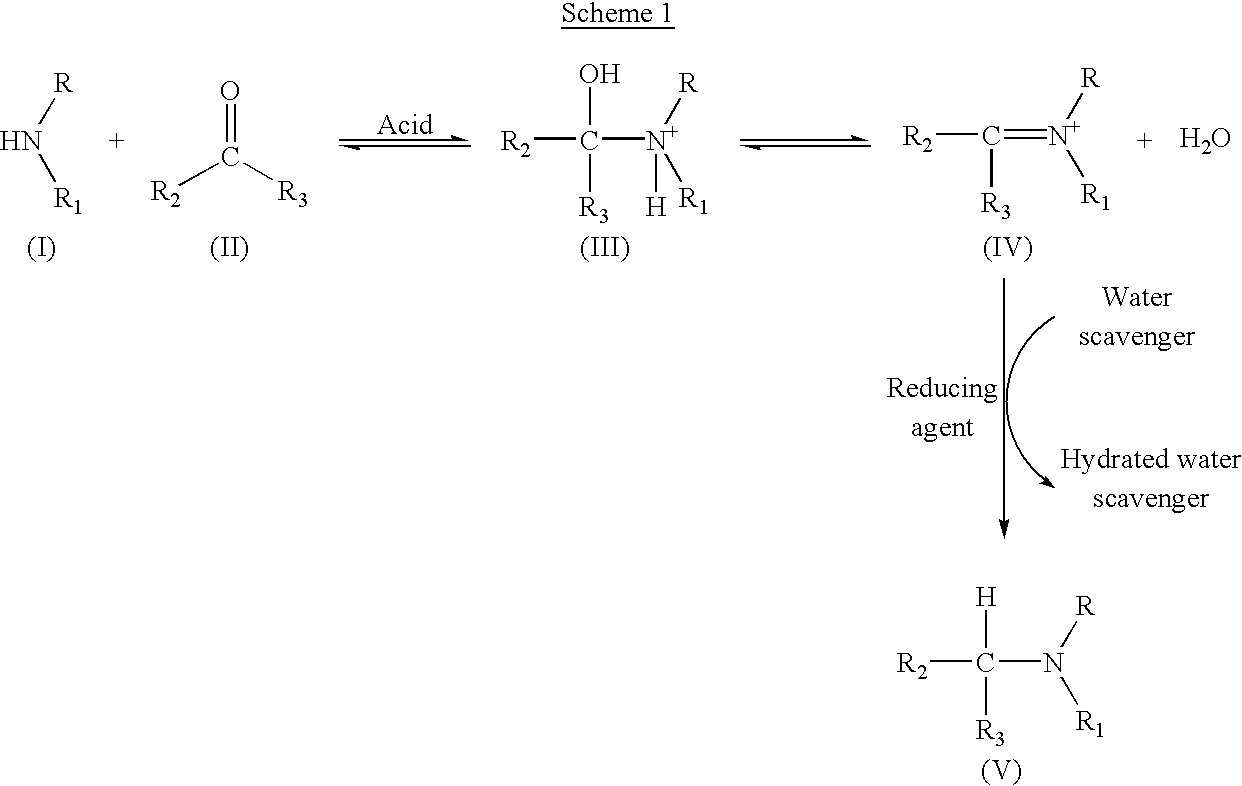
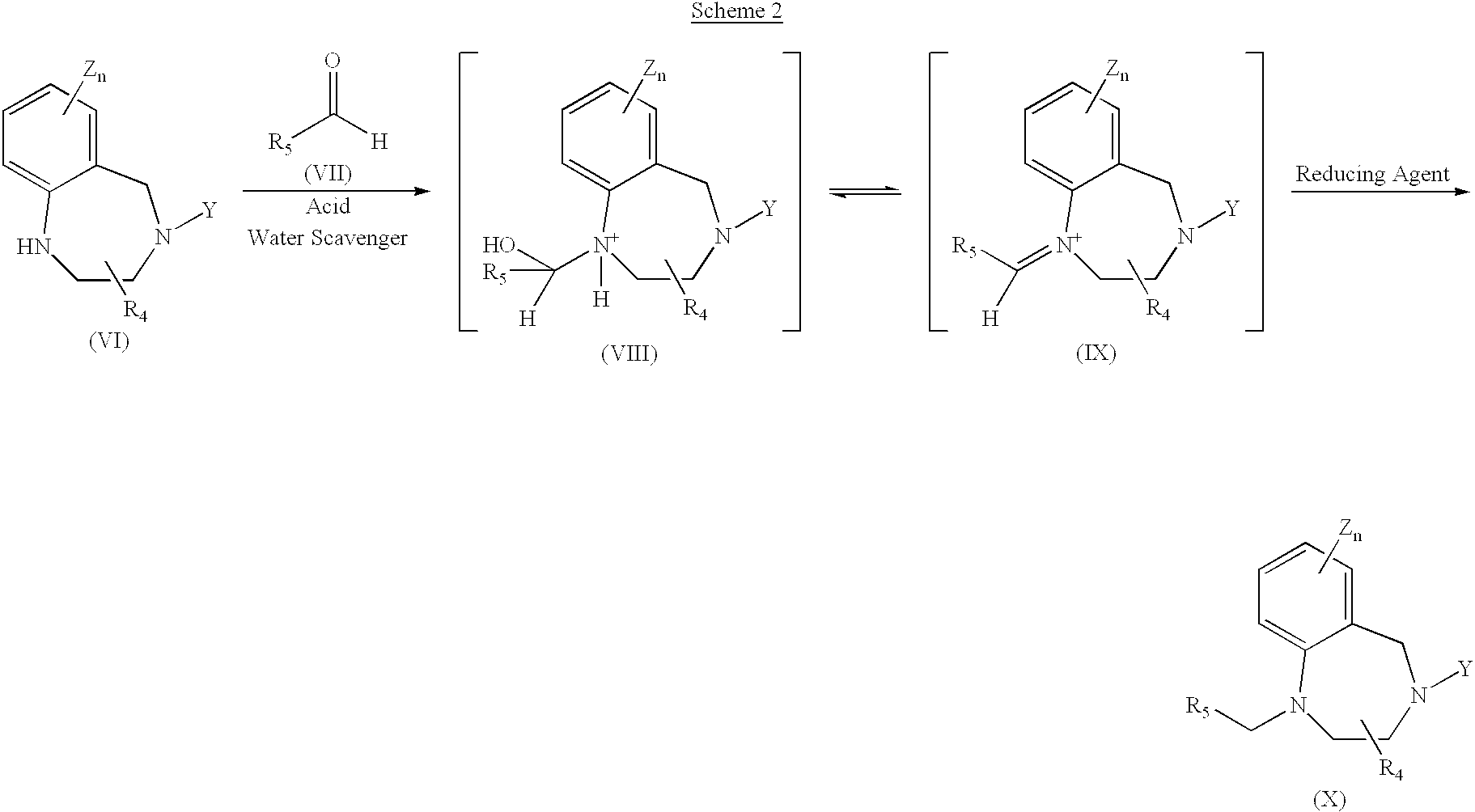
InactiveUS6949642B2Extend cycle timeIncrease costOrganic compound preparationPreparation by reductive alkylationScavengerFarnesyl Protein Transferase
A process for the production of tertiary amines by reductive amination of carbonyl compounds with secondary amines in the presence of a water scavenger, preferably trifluoroacetic acid anhydride, is disclosed. This process has applications in the preparation of imidazole-containing benzodiazepines, which are inhibitors of farnesyl protein transferase.
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
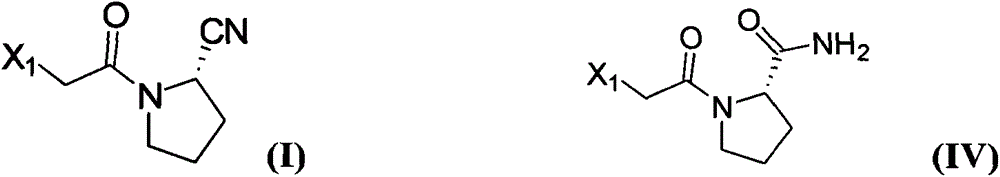
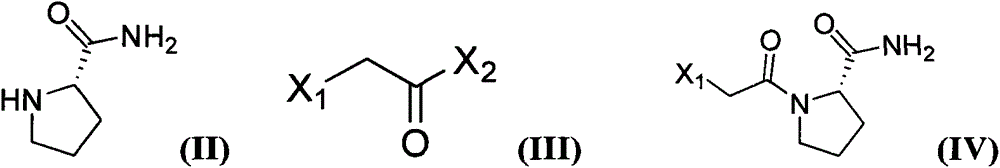
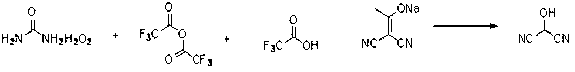
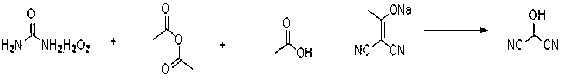
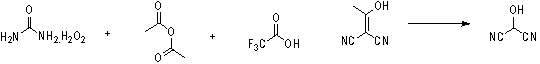
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000021.png)
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000031.png)
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000051.png)