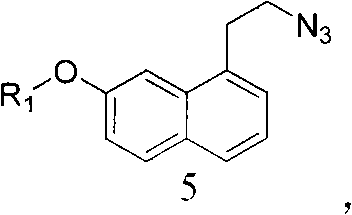
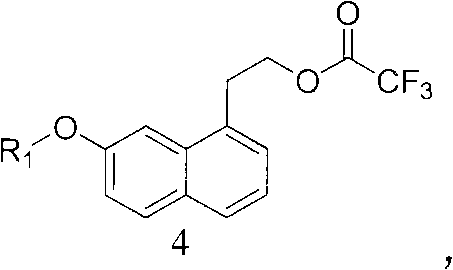
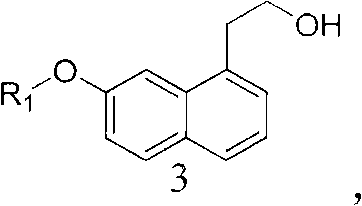
Intermediate for preparing agomelatine and relevant preparation method
A technology for use and phase transfer catalyst, applied in the field of agomelatine and its intermediate preparation, can solve the problems of difficult to realize industrialization, expensive starting materials, etc., and achieve high industrial application value, moderate reaction conditions, and reaction operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: Preparation of 1-bromo-7-methoxynaphthalene
[0078] Add 1-bromo-7-naphthol (5.6g, 0.025mol) successively in the 250ml four-neck round bottom flask, 100ml toluene, dimethyl sulfate (15.8g, 0.125mol) and tetramethylammonium bromide (0.25g, 0.0015mol), start mechanical stirring. 14.0 g of 50% KOH aqueous solution was added dropwise at 25-40°C. After dripping, continue to react. The reaction was monitored by TLC until the conversion of the starting material 1-bromo-7-naphthol was complete. After the reaction, 10ml of water was added, the temperature was raised to 55-60°C, and the temperature was kept for 1 hour. Separate layers, take the upper organic phase and discard the lower aqueous phase. The organic phase was washed successively with 5% aqueous sodium hydroxide and 5% aqueous sodium chloride. The solvent was evaporated to dryness to obtain 5.39 g of 1-bromo-7-methoxynaphthalene as a colorless waxy solid, with a yield of 90.94%.
[0079] 1 H NMR (...
Embodiment 2
[0081] Embodiment 2: Preparation of 1-bromo-7-methoxynaphthalene
[0082] In the 1000ml four-neck round bottom flask, add 1-bromo-7-naphthol (22.3g, 0.10mol) successively, 400ml toluene, dimethyl sulfate (63.2g, 0.125mol) and tetrabutylammonium bromide (1.0g, 0.003mol) to start the mechanical stirring. 56.0 g of 50% KOH aqueous solution was added dropwise at 10-15°C. After dripping, continue to react. The reaction was monitored by TLC until the conversion of the starting material 1-bromo-7-naphthol was complete. After the reaction, 100ml of water was added, the temperature was raised to 55-60°C, and the temperature was kept for 1 hour. Separate layers, take the upper organic phase and discard the lower aqueous phase. The organic phase was washed successively with 5% aqueous sodium hydroxide and 5% aqueous sodium chloride. The solvent was evaporated to dryness to obtain 22.52 g of 1-bromo-7-methoxynaphthalene as a colorless waxy solid, with a yield of 95.0%.
Embodiment 3
[0083] Embodiment 3: Preparation of 1-bromo-7-propoxynaphthalene
[0084] In the 250ml four-neck round bottom flask, add 1-bromo-7-naphthol (5.6g, 0.025mol) successively, 100ml toluene, dipropyl sulfate (22.8g, 0.125mol) and tetrapropylammonium bromide (0.4g, 0.0015mol), start mechanical stirring. 14.0 g of 50% KOH aqueous solution was added dropwise at 25-40°C. After dripping, continue to react. The reaction was monitored by TLC until the conversion of the starting material 1-bromo-7-naphthol was complete. After the reaction, 10ml of water was added, the temperature was raised to 55-60°C, and the temperature was kept for 1 hour. Separate layers, take the upper organic phase and discard the lower aqueous phase. The organic phase was washed successively with 5% aqueous sodium hydroxide and 5% aqueous sodium chloride. The solvent was evaporated to dryness to obtain 6.08 g of 1-bromo-7-propoxynaphthalene as a colorless waxy solid, with a yield of 91.83%.
[0085] MS (EI + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com