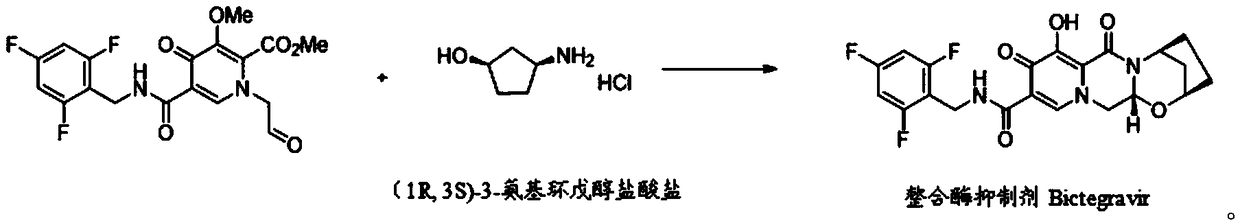
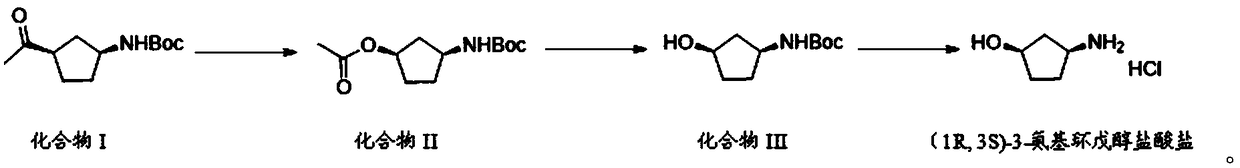
Preparation method of (1R,3S)-3-amino cyclopentanol hydrochloride
A technology of aminocyclopentanol hydrochloride and isopropanol, applied in the field of preparation of -3-aminocyclopentanol hydrochloride, can solve the problems of restricting industrial application, high application risk factor and high operation requirements, and achieves suitable Large-scale production, good process stability, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a preparation method of (1R,3S)-3-aminocyclopentanol hydrochloride, comprising the following steps:
[0034] (1) In a protective atmosphere, mix compound I, carbamide peroxide and an organic solvent, add trifluoroacetic anhydride to the resulting mixture, and carry out an oxidation reaction to obtain a mixture of compound II and compound II';
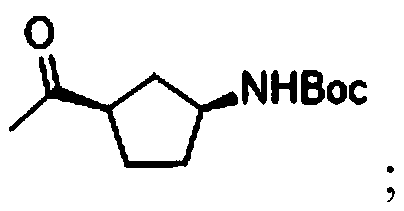
[0035] The structural formula of the compound I is:
[0036]
[0037] The structural formula of the compound II is:
[0038]
[0039] The structural formula of the compound II' is:
[0040]
[0041] (2) mixing the mixture of compound II and compound II' in the step (1), alkali solution and organic solvent, and performing a hydrolysis reaction to obtain compound III;
[0042] The structural formula of the compound III is:
[0043]
[0044] (3) In a protective atmosphere, the acid chloride compound and the compound III in the step (2) are sequentially added to isopropanol for a deprotection rea...
Embodiment 1
[0081](1) Under the protection of nitrogen, put 600mL tetrahydrofuran into the reaction flask, turn on the stirring device, put in 60.1g carbamide peroxide, 60g compound I, control the temperature to 0°C, and add 138.5g trifluoroacetic anhydride dropwise to the resulting mixed material After the dropwise addition was completed, the oxidation reaction was carried out with insulation for 7 hours; after the oxidation reaction was monitored by GC, the pH value of the obtained system was adjusted to 7.5 with a sodium carbonate solution with a mass concentration of 30%, and the temperature control was <10°C. layer, the obtained aqueous phase was extracted with ethyl acetate, the obtained organic phase was washed successively with 180 mL of 5% sodium thiosulfate solution and 180 mL of saturated brine, and the obtained organic phase was dried with anhydrous sodium sulfate. After filtration, the obtained organic phase was concentrated under reduced pressure, and the obtained concentrate...
Embodiment 2
[0086] (1) Prepare the mixture of compound II and compound II' according to the method of step (1) in Example 1, the difference is that the temperature when trifluoroacetic anhydride is added dropwise is -20°C, and the temperature of the oxidation reaction is -20°C °C, the time of the oxidation reaction was 18h; the total purity of the mixture of compound II and compound II' obtained by final GC detection was 82.0%;
[0087] (2) Compound III was prepared according to the method of step (2) in Example 1, the difference being that an aqueous solution of sodium hydroxide (prepared by dissolving 25.8g of sodium hydroxide in 180mL of water) was added dropwise at 0°C, and finally Obtained 55.8g of compound III, GC detection showed that the purity was 90.5%, and the yield was 95.3%;
[0088] (3) Prepare (1R,3S)-3-aminocyclopentanol hydrochloride according to the method of step (3) in Example 1, the difference is that 71.6g propionyl chloride is used as the acid chloride compound, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com