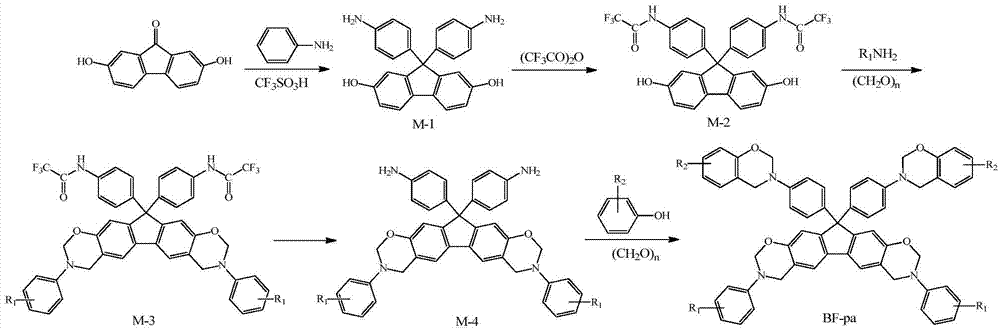
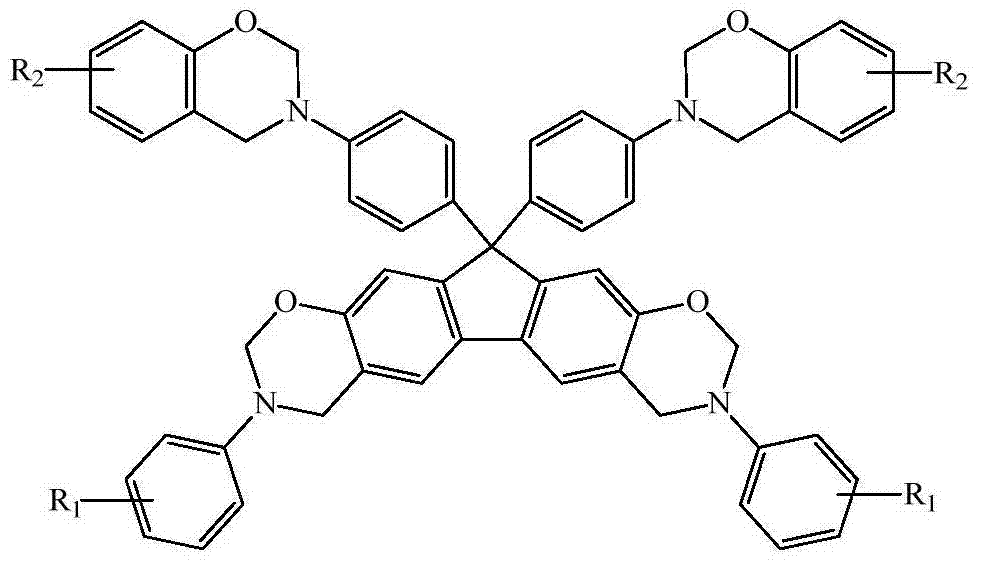
N-full aromatic hydrocarbyl bisphenol-diamine tetrafunctional fluorene-based benzoxazine and preparation method thereof
A fully aromatic hydrocarbon-based bisphenol, four-functionality technology, applied in the direction of organic chemistry, can solve problems such as difficult to obtain target products, achieve the effect of expanding application fields, excellent thermal stability, and improving processing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of 2,7-dihydroxy-9,9-bis-(4-aminophenyl)fluorene
[0035] Add 0.05mol 2,7-dihydroxy-9-fluorenone, 0.40mol aniline and 0.015mol trifluoromethanesulfonic acid to a four-necked flask with a stirring rotor, a condenser tube, a thermometer and a gas inlet in sequence, and feed in nitrogen, React at 150°C for 10 h, then cool to room temperature, pour the product into 100 mL of 5 g / L sodium hydroxide ethanol solution, filter the precipitate, wash with ethanol, and dry in vacuum to obtain 2,7-dihydroxy-9,9 - Bis-(4-aminophenyl)fluorene (M-1), the yield is 92.3%.
[0036] (2) Synthesis of bis-trifluoroacetanilide-based bisphenol fluorene monomer
[0037] Add 100mL tetrahydrofuran and 0.05mol M-1 into a three-neck flask equipped with a stirring rotor and a condenser tube, stir in an ice-water bath for 15min, then slowly add 0.15mol trifluoroacetic anhydride dropwise, after the dropwise addition, continue the reaction at room temperature for 4h , remove tetrahydrof...
Embodiment 2
[0047] Except that 0.045mol potassium borohydride in the synthesis step (4) was changed to 0.018mol potassium carbonate, and the reaction time was changed from 7h to 16h, other conditions were the same as in Example 1, and finally BF-pa-1 was obtained, and the total yield of the product was 44.5% ( The calculation is based on the yield of synthesis steps 2 to 5, the same below).
Embodiment 3
[0049] Except that 0.045mol potassium borohydride in the synthesis step (4) was changed to 0.036mol ammonia water, the phenol in the synthesis step (5) was changed to cardanol, and the organic solvent was changed from 4mL chlorobenzene and 8mL xylene to 2mL chlorobenzene and 10mL dioxane ring, the reaction temperature was changed from 140°C to 110°C, and the reaction time was changed from 4h to 24h, other conditions were the same as in Example 1, and finally aniline-cardanol-derived N-fully aromatic hydrocarbon-based bisphenol-bisamine tetrafunctional Fluorenylbenzoxazine monomer (BF-pa-2), T m The temperature was 83°C, and the total yield of the product was 45.2%.
[0050] 1 H NMR: 6.58~7.43 (m, 28H, Ar-H), 5.74~5.83 and 4.96~5.04 (m, =CH- and -CH=CH 2 ), 5.41 and 5.34 (d, 8H, O-CH 2 -N), 4.71 and 4.55 (d, 8H, Ar-CH 2 -N), 2.73~2.77 (m, =CH-CH 2 -CH=), 2.41~2.47 (m, Ar-CH 2 -), 2.21~2.25 (m, =CH-CH 2 -), 1.25 ~ 1.97 (m, -CH 2 -), 0.82~0.85 (m, -CH 3 ) (Note: Except f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com