Clean and high-conversion-rate preparing method of 2,2,2-trifluroacetophenone
A trifluoroacetophenone, high conversion technology, applied in the preparation of organic compounds, the preparation of carbon-based compounds, chemical instruments and methods, etc., can solve the problems of low yield, difficult handling, high solvent toxicity, etc. Good selectivity, reduce the generation of impurities, and improve the effect of nucleophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
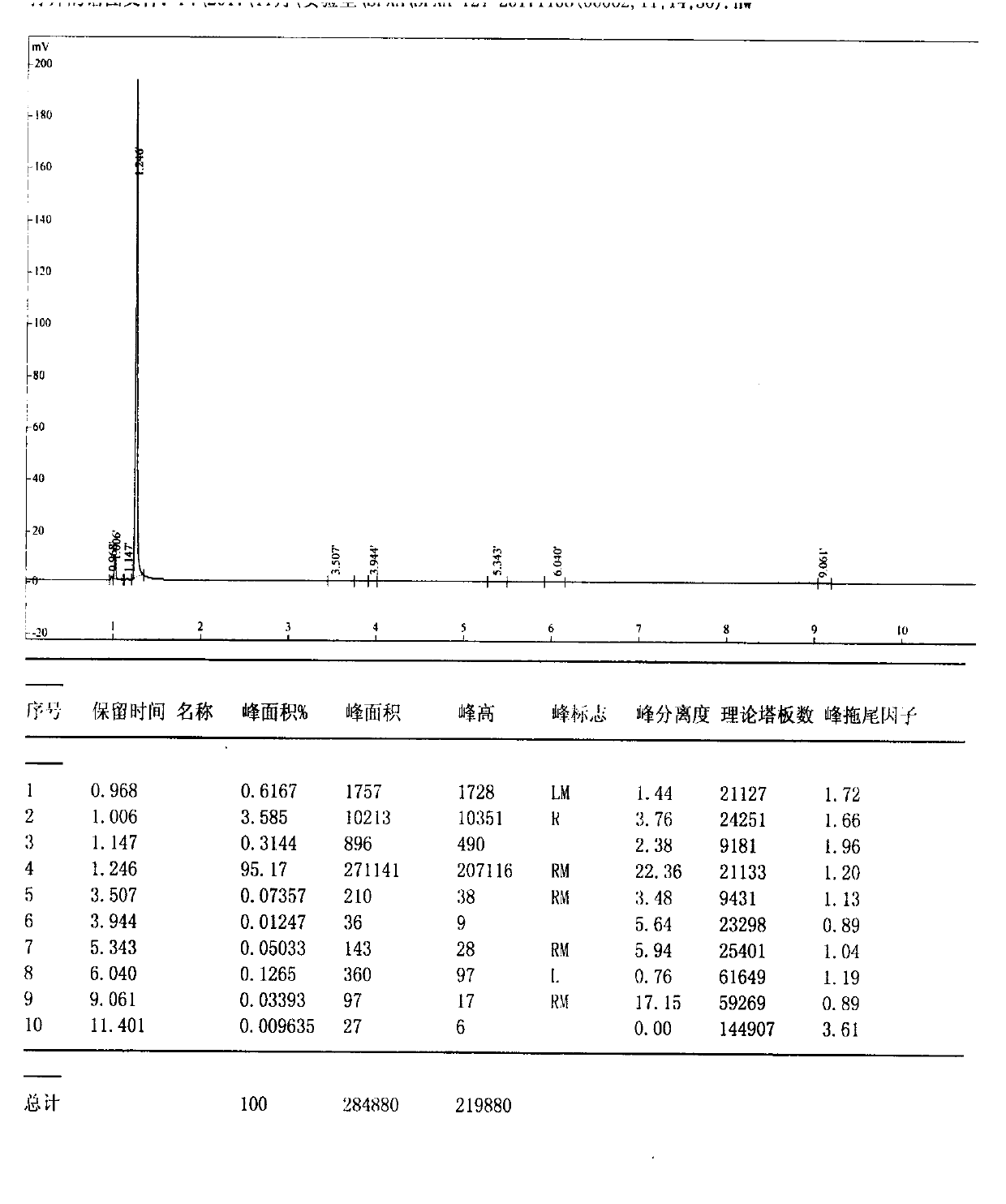
Image
Examples
Embodiment 1
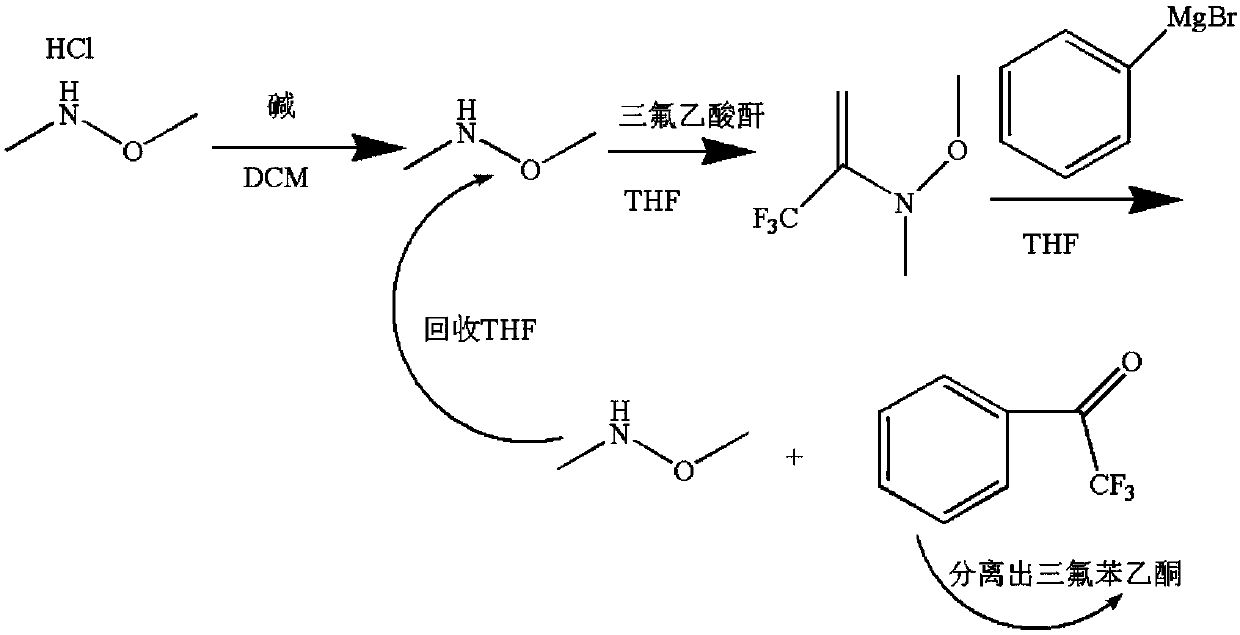
[0026] Such as figure 1 The preparation method of described 2,2,2-trifluoroacetophenone clean high conversion rate, comprises the following steps:
[0027] S1. Add 1mol (97.5g) N,O-dimethyl hydroxylamine hydrochloride, 1mol (149.1g) triethanolamine, 600ml dichloromethane into the reaction vessel, and stir the reaction for 1h at room temperature to obtain free N,O- Dimethylhydroxylamine;
[0028] S2. At 0°C, add dropwise 150ml of dichloromethane solution containing 1mol (210g) trifluoroacetic anhydride to the free N,O-dimethylhydroxylamine obtained in step 2, dropwise for 1.5h, keep warm and continue the reaction 1.5h, after the reaction was completed, add 200ml of water to dilute and separate the layers. After the aqueous phase was washed twice with 300ml of dichloromethane solution, the organic layer was combined, and the organic layer was washed twice with 400ml of water and dried over anhydrous sodium sulfate. Recover to dryness at -35°C to obtain N,O-dimethyltrifluoroami...
Embodiment 2
[0033] Such as figure 1 The preparation method of described 2,2,2-trifluoroacetophenone with clean and high conversion rate is characterized in that it comprises the following steps:
[0034] S1. Add 1mol (97.5g) N,O-dimethyl hydroxylamine hydrochloride, 1.05mol (194.5g) tri-n-butylamine, 600ml dichloromethane into the reaction vessel, stir and react for 2h at room temperature to obtain free N , O-dimethylhydroxylamine;
[0035] S2. At 15°C, add 1.03mol (215g) of trifluoroacetic anhydride in 150ml of dichloromethane dropwise to the free N,O-dimethylhydroxylamine obtained in step 2. Add dropwise for 1 hour, and keep warm for 1 hour. After the reaction, add 200ml of water to dilute and separate layers. After the aqueous phase is washed twice with 300ml of dichloromethane solution, the organic layer is combined, and the organic layer is washed twice with 400ml of water and dried over anhydrous sodium sulfate. Recover to dryness at ℃ to obtain N, O-dimethyl trifluoroamide to be ...
Embodiment 3
[0040] Such as figure 1 The preparation method of described 2,2,2-trifluoroacetophenone clean high conversion rate, comprises the following steps:
[0041]S1. Add 1 mol (97.5 g) N, O-dimethyl hydroxylamine hydrochloride, 1.02 (152.5 g) mol triethanolamine, 600 ml dichloromethane into the reaction vessel, and stir the reaction for 1 h at room temperature to obtain free N, O - Dimethylhydroxylamine;
[0042] S2. At 0°C, add 1.05mol (220g) of trifluoroacetic anhydride in 150ml of dichloromethane dropwise to the free N,O-dimethylhydroxylamine obtained in step 2, dropwise for 1.5h, and keep warm to continue the reaction 2h, after the reaction is over, add 200ml of water to dilute and separate the layers. After the aqueous phase is washed twice with 300ml of dichloromethane solution, the organic layer is combined, and the organic layer is washed twice with 400ml of water and dried over anhydrous sodium sulfate. Recover to dryness at 35°C to obtain N,O-dimethyltrifluoroamide to be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com