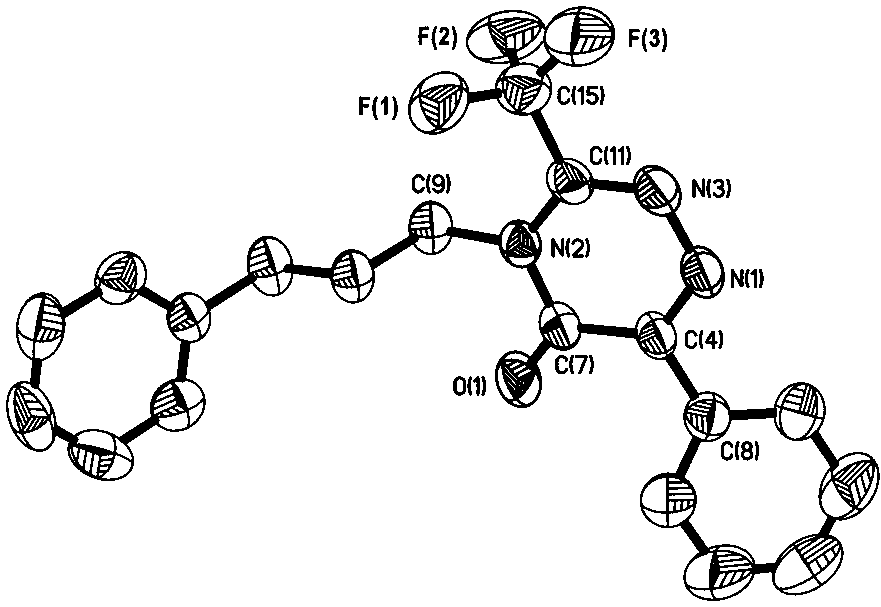
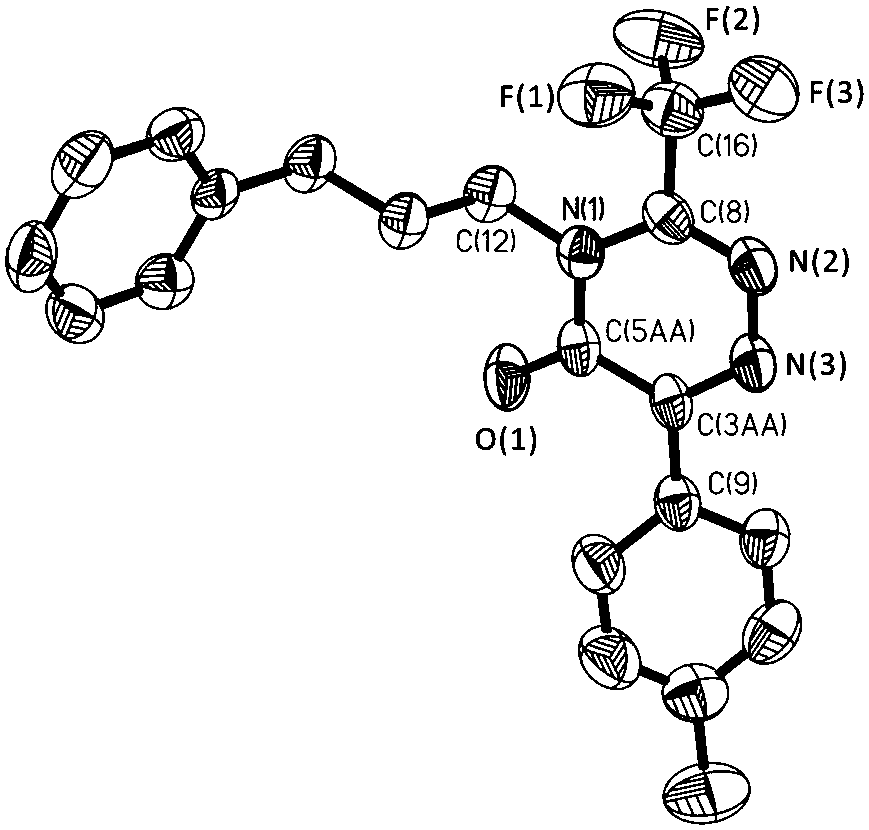
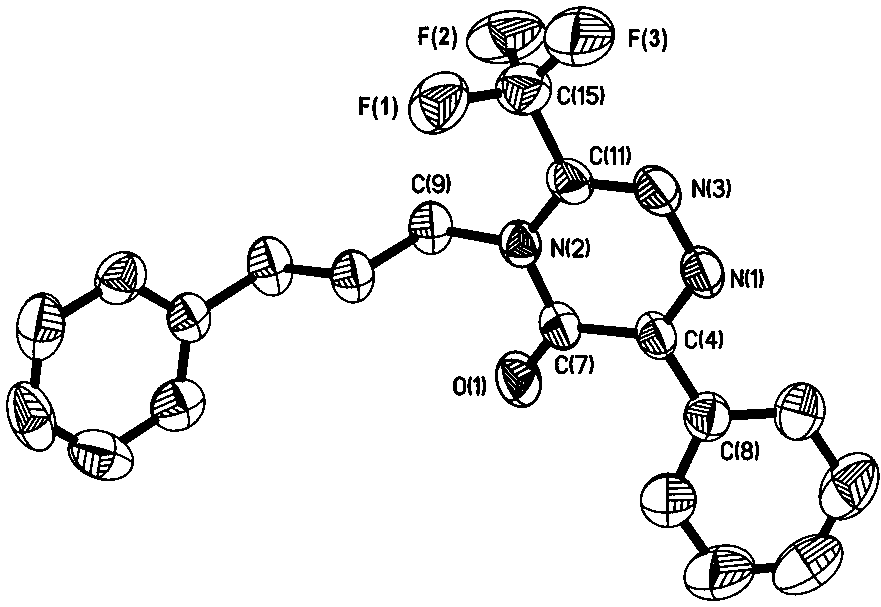
Method for synthesizing trifluoromethyl-1,2,4-triazinone compound through copper catalyzed synthesis
A technology of trifluoromethyl and triazinone, which is applied in the field of organic fluorine chemical synthesis and achieves the effects of good adaptability, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a 5 mL reaction tube, add 0.025 mmol cuprous iodide, 1 mL tetrahydrofuran, 0.5 mmol phenylacetylene, 0.65 mmol 1-azido-3-benzene Propane, 0.75 mmol trifluoroacetic anhydride, finally add 1 mmol triethylamine, stir and react in a closed system at room temperature for 9 h, filter with 100-200 mesh silica gel, rinse with dichloromethane, combine the organic phases, and spin The organic solvent was removed by evaporation; the obtained crude product was eluted with n-pentane and ethyl acetate by silica gel column chromatography to obtain 6-phenyl-(3'-phenylpropyl)-3-(trifluoromethyl) -1,2,4-triazine-5(4 H )-ketone (90% isolated yield). 1 H NMR (400 MHz, CDCl 3 ) δ 8.28 (d, J = 7.8 Hz, 2H), 7.54 (t,1H), 7.47 (t, J = 7.5 Hz, 2H), 7.29 (t, J = 7.4 Hz, 2H), 7.20 (t, J = 6.8 Hz,3H), 4.08 (t, 2H), 2.76 (t, J = 7.6 Hz, 2H), 2.20 – 2.01 (dt, 2H). 19 F NMR (376 MHz, CDCl 3 ) δ -65.5 (s, 3F).
Embodiment 2
[0023] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a 5 mL reaction tube, add 0.025 mmol cuprous iodide, 1 mL tetrahydrofuran, 0.5 mmol 4-tert-butylphenylacetylene, 0.65 mmol 1-azide Base-3-phenylpropane, 0.75mmol trifluoroacetic anhydride, finally add 1 mmol triethylamine, stir and react in a closed system at room temperature for 9 h, filter with 100-200 mesh silica gel, wash with dichloromethane, and combine Organic phase, and then the organic solvent was removed by rotary evaporation; the obtained crude product was eluted with n-pentane and ethyl acetate to obtain 6-(4'-(tert-butyl)phenyl)-4-( 3''-phenylpropyl)-3-(trifluoromethyl)-1,2,4-triazine-5(4 H )-ketone (86% isolated yield). 1 H NMR (400 MHz, CDCl 3 ) δ 8.30 (d, J = 8.6 Hz, 2H), 7.56 (d, J = 8.6 Hz, 2H), 7.34 (t, 2H), 7.25 (t, J = 6.2 Hz,3H), 4.13 (t, 2H), 2.81 (t, J = 7.6 Hz, 2H), 2.26 – 2.03 (dt, 2H), 1.41 (s,9H). 19 F NMR (376 MHz, CDCl 3 ) δ -65.5 (s, 3F).
Embodiment 3
[0025] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a 5 mL reaction tube, add 0.025 mmol cuprous iodide, 1 mL tetrahydrofuran, 0.5 mmol 4-ethynyl phenpentyl ether, 0.65 mmol 1-azide Base-3-phenylpropane, 0.75mmol trifluoroacetic anhydride, finally add 1 mmol triethylamine, stir and react in a closed system at room temperature for 9 h, filter with 100-200 mesh silica gel, wash with dichloromethane, and combine Organic phase, and then the organic solvent was removed by rotary evaporation; the obtained crude product was eluted with n-pentane and ethyl acetate by silica gel column chromatography to obtain 6-(4'-(pentyloxy)phenyl)-4-( 3''-phenylpropyl)-3-(trifluoromethyl)-1,2,4-triazine-5(4 H )-ketone (83% isolated yield). 1 H NMR (400 MHz, CDCl 3 ) δ 8.41 (d, J = 9.0 Hz, 2H), 7.32 (t, 2H), 7.24 (t, J = 5.8 Hz, 3H), 6.99 (d, J = 9.0 Hz,2H), 4.16 – 3.91 (m, 4H), 2.78 (t, J = 7.6 Hz, 2H), 2.12 (dt, J = 15.6, 7.8Hz, 2H), 1.93 – 1.73 (m, 2H), 1.57 – 1.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com