Synthesizing method of 2-pyridine methyl sulfide and synthesizing process of related drugs
A technology of picolyl sulfide and synthesis method, which is applied in the direction of organic chemistry, can solve problems such as environmental pollution of halogenated substances, and achieve the effects of good applicability, high yield, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
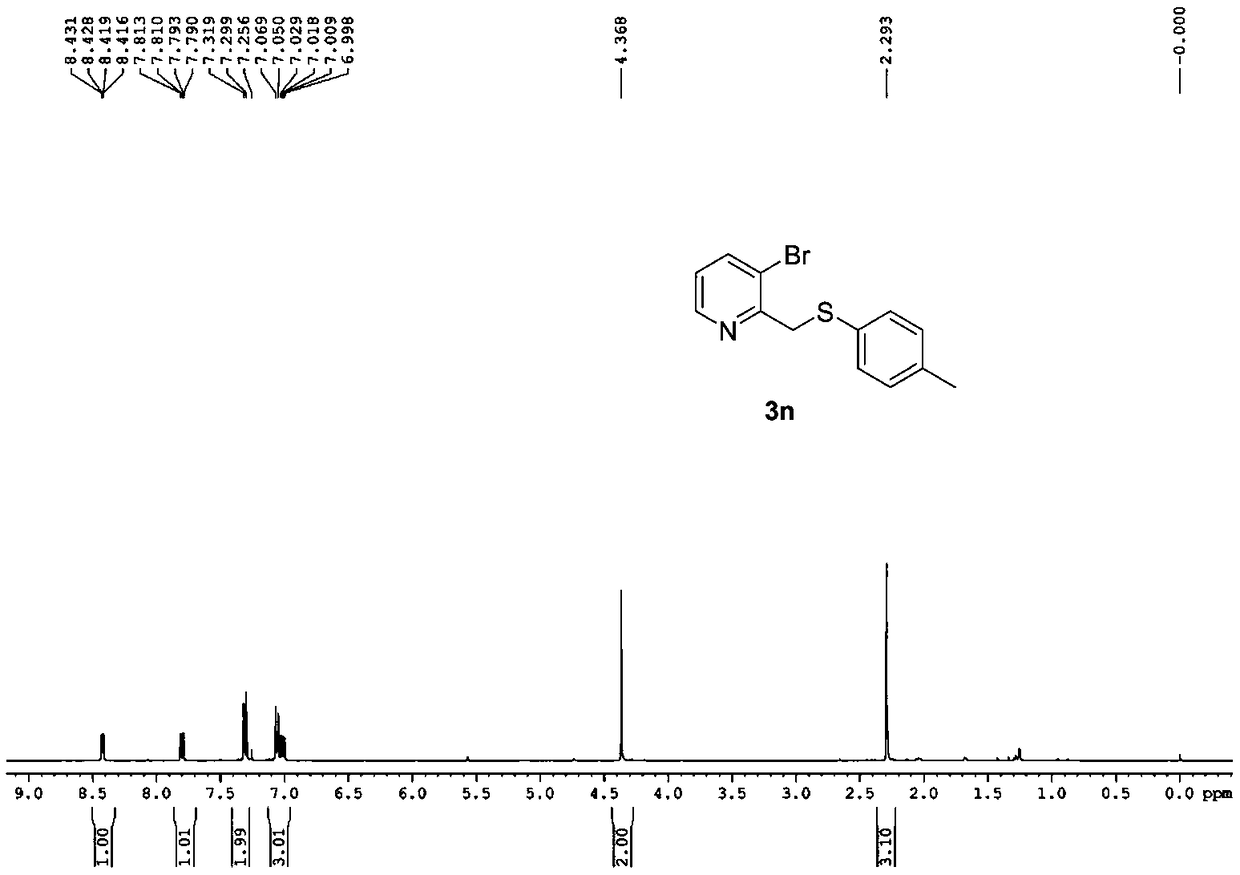
[0038] 2-Methylpyridine nitroxide (507mg, 4.65mmol) was dissolved in ethyl acetate (12ml) at room temperature, then trifluoroacetic anhydride (2.44g, 11.6mmol) was added. The reaction was refluxed for two hours. The completion of the reaction was monitored by TLC, and the reaction solution was concentrated to obtain the trifluoroacetate intermediate, which was directly used for the next step. The reaction intermediate was dissolved in toluene (6ml), then 4-methylthiophenol (577mg, 4.65mmol), tetrabutylammonium bromide (300mg, 0.93mmol) were added, and the reaction was heated under reflux for five hours, and the reaction was monitored by TLC At the end, add saturated sodium carbonate aqueous solution to the reaction solution to adjust the pH to 7-8, then separate the liquids, extract the aqueous phase with ethyl acetate three times, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate, and use PE-PE / EA (50:1~1:3) was used as ...
Embodiment 2
[0040]
[0041] The synthetic method of embodiment 2 is the same as above-mentioned synthetic general method.
[0042] Reaction yield: 71%; Structural parameters: 1 H NMR (400MHz, CDCl 3 )δ8.54(d, J=4.4Hz, 1H), 7.62-7.58(m, 1H), 7.32(d, J=7.6Hz, 1H), 7.16-7.7.13(m, 4H), 6.98(t , J=4.0 Hz, 1H), 4.26(s, 2H), 2.28(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ157.4,148.9, 138.2,136.3,135.3,129.8,128.4,126.9,126.1,122.7,121.7,40.1,21.0.HRMS (+ESI-TOF)m / z:[M+H] + Calcd for C 13 h 14 NS 216.0841; Found 216.0840.
Embodiment 3
[0044]
[0045] The synthetic method of embodiment 3 is the same as above-mentioned synthetic general method.
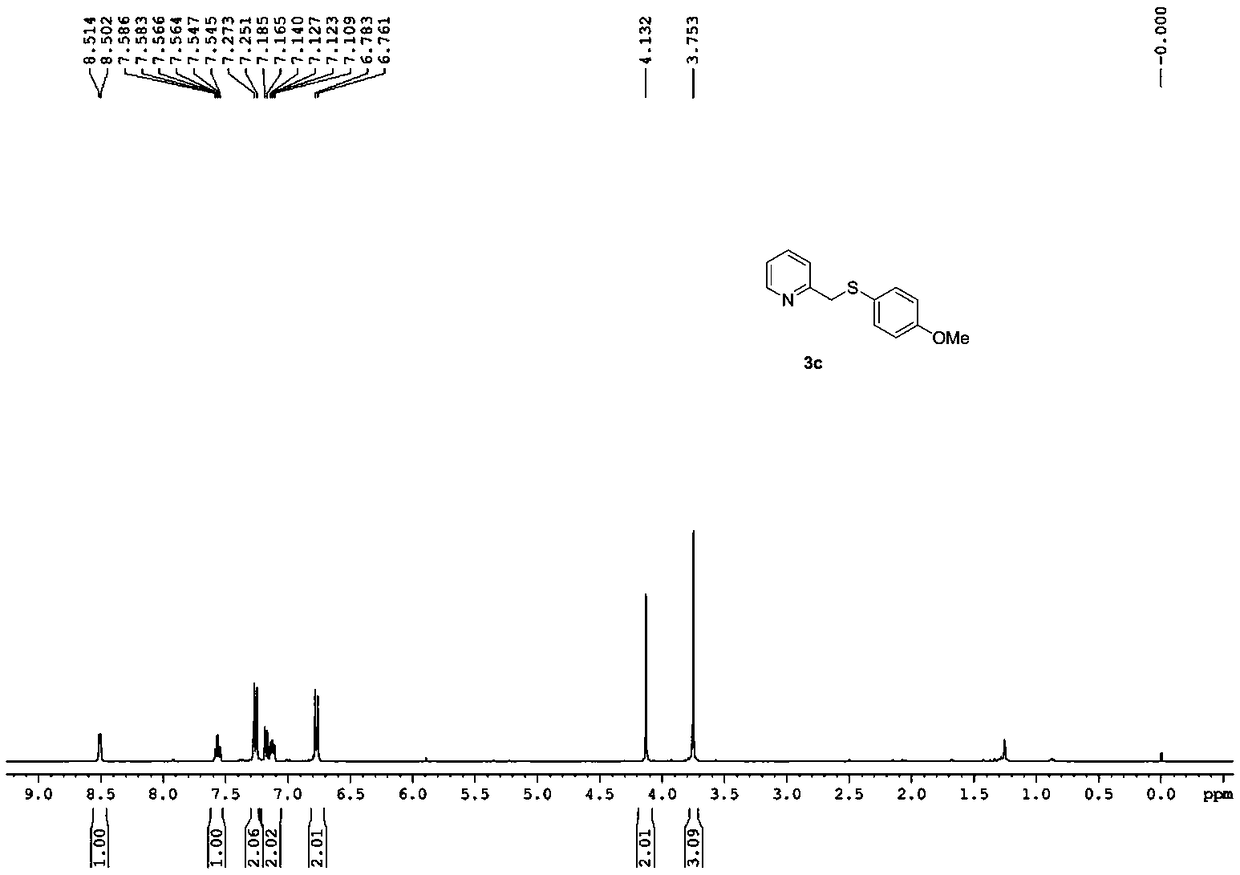
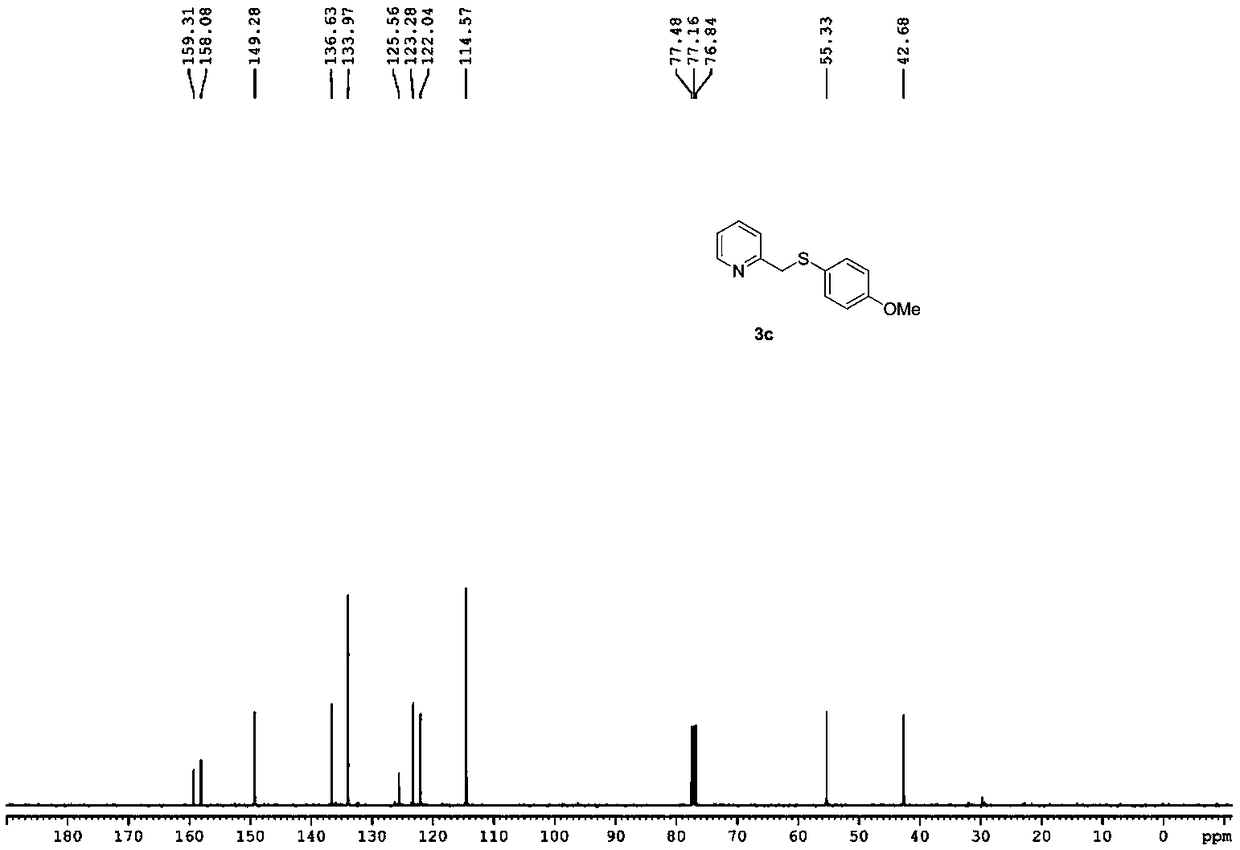
[0046] Reaction yield: 70%; Structural parameters: 1 H NMR (400MHz, CDCl 3 )δ8.51(d, J=4.8Hz, 1H), 7.59-7.55(m, 1H), 7.26(d, J=8.8Hz, 2H), 7.18(d, J=8.0Hz, 1H), 7.13( dd,J=5.2,6.8Hz,1H),6.77(d,J=8.8Hz,2H),4.13(s,2H),3.75(s,3H). 13 C NMR (100 MHz, CDCl 3)δ159.3, 158.1, 149.3, 136.6, 134.0, 125.6, 123.3, 122.0, 114.6, 55.3, 42.7.HRMS(+ESI-TOF)m / z:[M+H] + Calcd for C 13 h 14 NOS 232.0791; Found 232.0777.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com