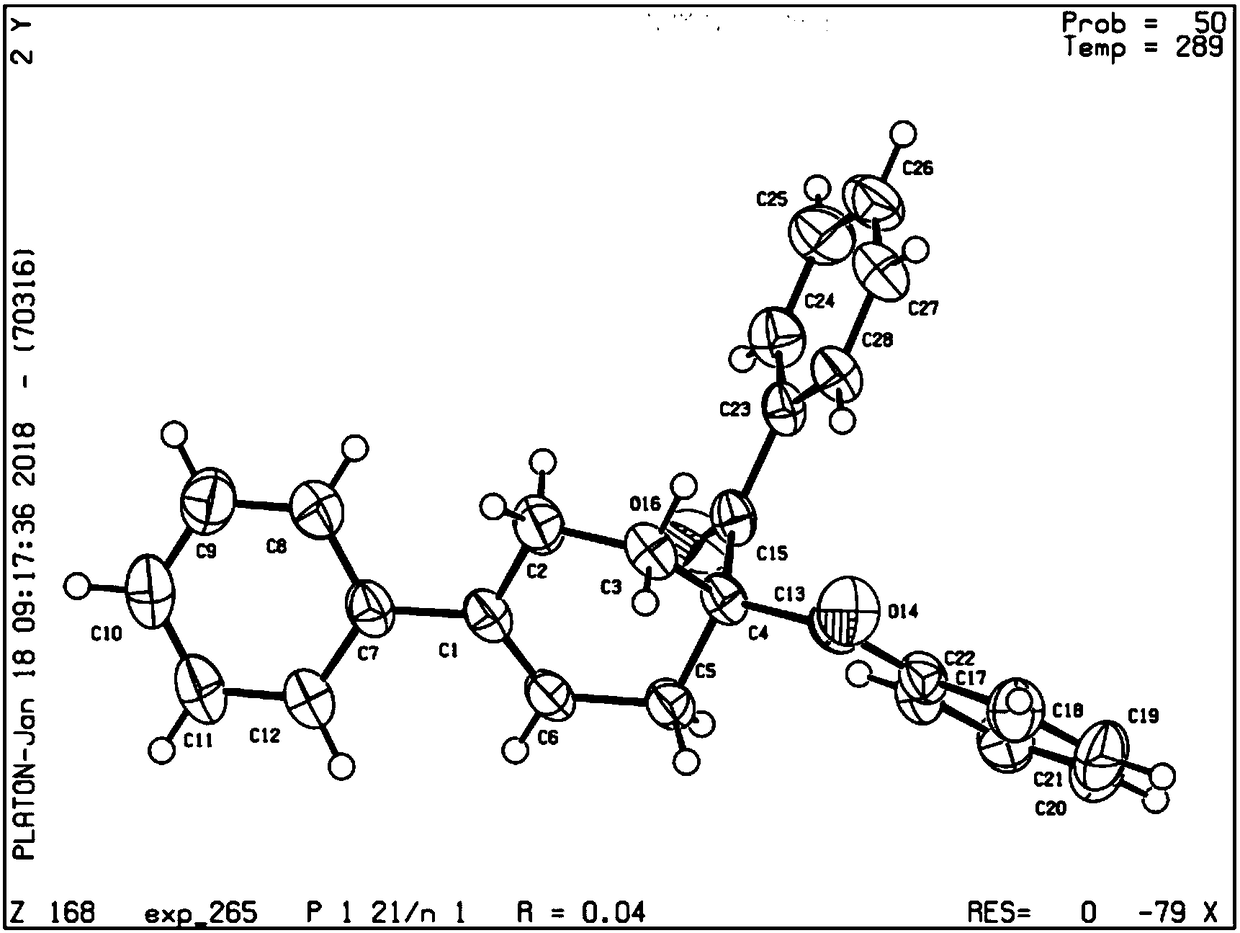
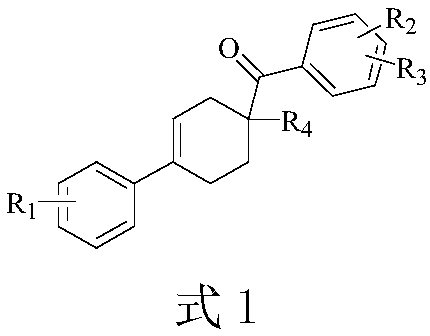
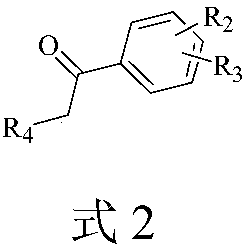
Method for synthesizing 1,4-disubstituted cyclohexene derivative
A synthesis method and technology of cyclohexene, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve problems such as difficult to obtain yield, difficult to obtain single, poor selectivity, etc., to achieve a wide range of applications , strong site selectivity, and the effect of avoiding the use of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The following examples are intended to further illustrate the content of the present invention, rather than limit the protection scope of the claims of the present invention.
[0043] All reactions were performed in Schlenk tubes unless otherwise stated.
[0044] All reaction stock solvents were obtained from commercial sources and used without further purification.
[0045] Product separation adopts silica gel chromatographic column, silica gel (particle size 300 mesh-400 mesh).
[0046] 1H NMR (400MHz), 13C NMR (100MHz) and 19F NMR (376MHz) detection adopts Bruker ADVANCEIII spectrometer, with CDCl 3 As the solvent, TMS is used as the internal standard, the chemical shift is in parts per million (ppm), and 0.0 ppm of tetramethylsilane is used as the reference shift. The following abbreviations (or combinations thereof) are used to explain multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. The unit of the coupling constant J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com