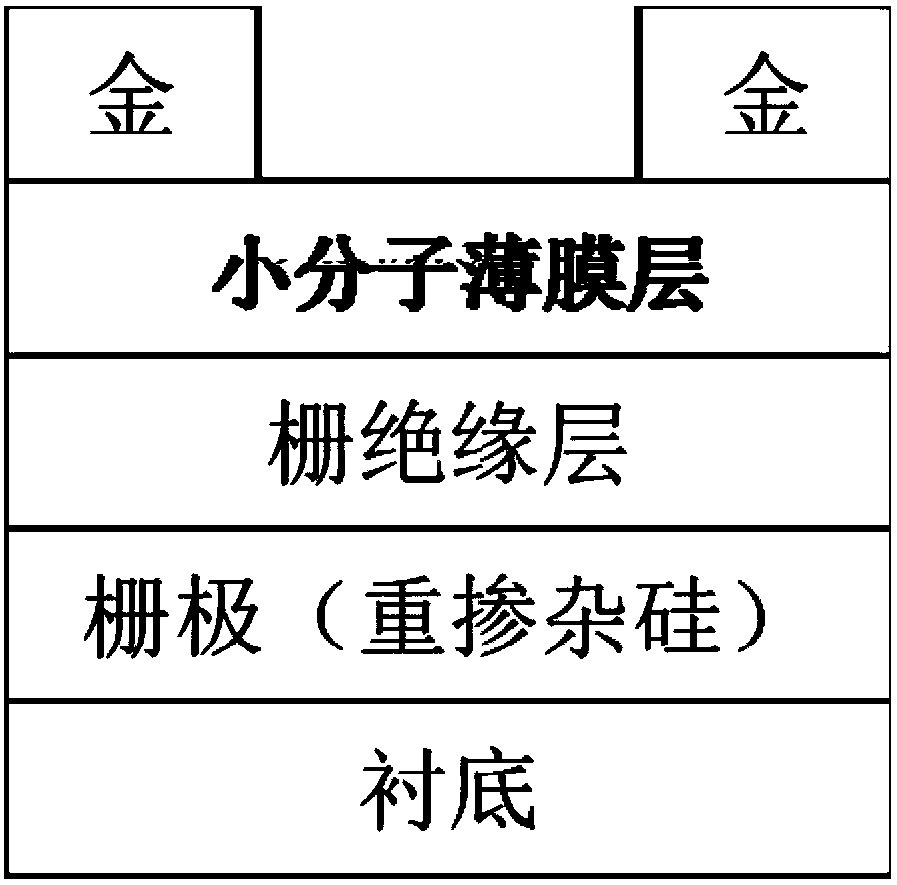
Polycyclic aromatic hydrocarbon organic semiconductor material and preparation method and application thereof
A technology of organic semiconductors and polycyclic aromatic hydrocarbons, applied in the field of polycyclic aromatic hydrocarbons organic semiconductor materials and their preparation, can solve the problems of long synthesis steps, difficult synthesis, cumbersome operation, etc., and achieve easy separation, less by-products, and high mobility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
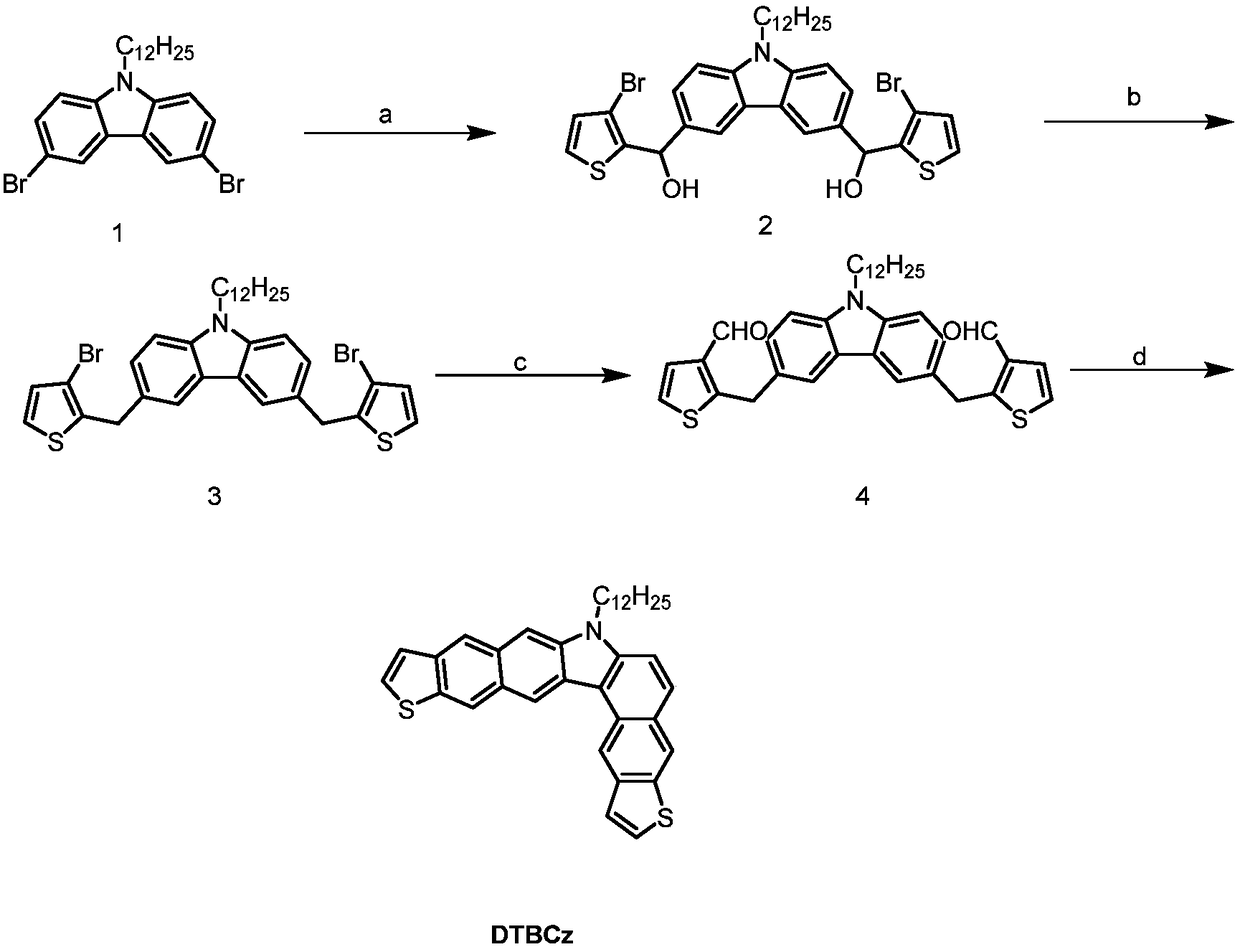
[0043] This example provides a method for preparing an angular molecular compound DTBCz with carbazole as the nucleus, and its synthetic route is as follows: figure 2 As shown, the finally obtained angular molecular structure with carbazole as the nucleus is as follows:
[0044]
[0045] The first step, take 14.9g (10mmol) of compound 1 (i.e. 3,6-dibromoalkylcarbazole), and dissolve compound 1 azole in 70mL of anhydrous tetrahydrofuran under the protection of nitrogen. Under protection, 8 mL (20 mmol) of butyl lithium with a concentration of 2.5 mol / L was added dropwise with a syringe, and kept stirring at low temperature for 1 h, and then 4.8 g (25 mmol) of 3-bromothiophene-2-carbaldehyde was added dropwise to continue the reaction. The reaction mixture was stirred at room temperature for 8 h and extracted with dichloromethane to obtain an organic phase. After drying the combined organic phases with sodium sulfate, the solvent was removed to obtain 3.6 g of a crude produc...
Embodiment 2
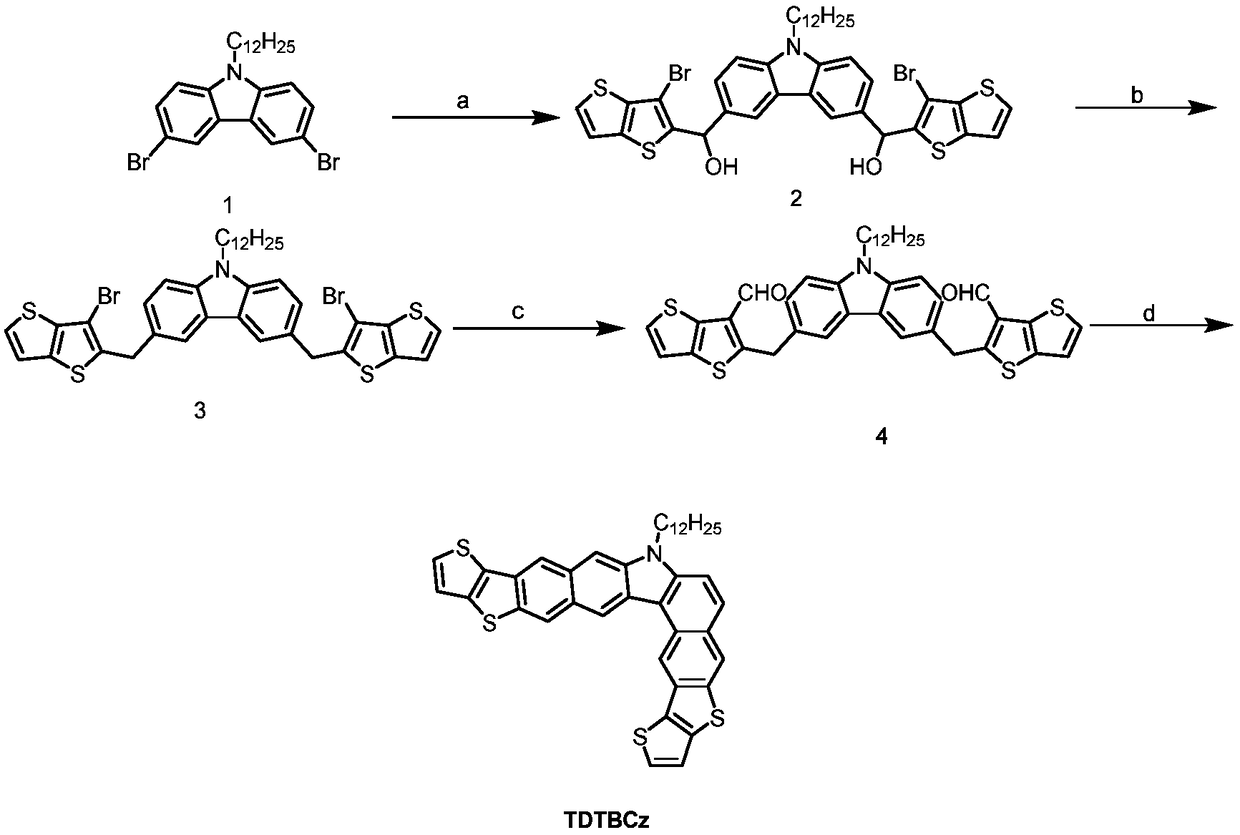
[0050] This example provides a preparation method for the angular molecular compound TDTBCz with carbazole as the nucleus, and its synthetic route is as follows image 3 As shown, the structural formula of the angular molecular compound TDTBCz with carbazole as the core finally obtained is:
[0051]
[0052] In the first step, 4.9g (10mmol) of compound 1 was dissolved in 70mL of anhydrous tetrahydrofuran under the protection of nitrogen, and 8mL of butyllithium solution with a concentration of 2.5mol / L was added using a syringe at a low temperature of -78°C and under the protection of nitrogen. (20mmol), kept stirring at low temperature for 1h, then added 6.18g (25mmol) of 3-bromo-2-formaldehyde-thiophene, and reacted to obtain a reaction mixture. After the reaction mixture was kept at room temperature for 8 h, the mixture was extracted with dichloromethane, the organic phase was taken, and the combined organic phase was dried over sodium sulfate. The solvent was then remo...
Embodiment 3
[0057] This example provides a method for preparing a horn-shaped molecular compound DTBCzC8 with carbazole as the nucleus, and its synthetic route is as follows Figure 4 As shown, the structural formula of the angular molecular compound DTBCzC8 with carbazole as the nucleus is obtained at last:
[0058]
[0059] In the first step, 5g (10mmol) of compound 1 was dissolved in 70mL of anhydrous tetrahydrofuran under the protection of nitrogen, and 8mL (20mmol) of butyllithium with a concentration of 2.5mol / L was added using a syringe under the protection of low temperature of -78°C. Keep stirring at low temperature for 1 h, then add 7.6 g (25 mmol) of 3-bromo-5-octylthiophene-2-carbaldehyde to obtain a reaction mixture. After the reaction mixture was stirred at room temperature for 8 h, it was extracted with dichloromethane, the organic phase was taken, and the combined organic phase was dried over sodium sulfate. The solvent was removed to obtain 3.3 g of the crude product ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com