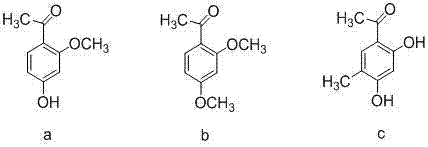
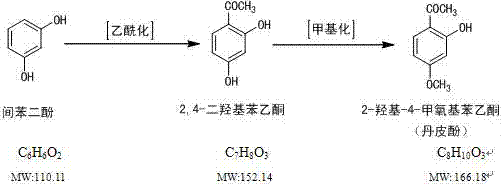
A new method for the synthesis and refinement of medicinal paeonol
A refining method and a technology for paeonol, applied in the field of drug synthesis, can solve the problems of difficulty in obtaining ideal purity and better crystal form, polluting the production environment, affecting production requirements, etc. The effect of partial oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Methylation reaction: 76.6g (0.5mol) 2,4-dihydroxyacetophenone, 250ml methyl Isobutyl ketone, 0.91g (0.0025mol) benzyltributylammonium bromide, 3.15g (0.025mol) sodium sulfite, 150ml purified water. Add 82.8 g (0.65 mol) of dimethyl sulfate at one time when the temperature of the water bath is raised to 45 °-50 °C under continuous stirring. After stirring evenly, add 25% potassium carbonate aqueous solution evenly dropwise into the reaction bottle from the constant pressure dropping funnel, and control the pH value of the reaction solution between 7.5 and 8.5 to carry out the methylation reaction. When the consumption of potassium carbonate reached 0.75 mol, regular samples were taken for TLC monitoring. If the raw material spots disappeared, it indicated that the reaction had reached the end point. Stop dripping and potassium carbonate solution, continue to stir for 15 minutes and then terminate the heating reaction, remove the water bath and cool down to below roo...
Embodiment 2
[0054] Embodiment 2 (the comparative example without reducing agent)
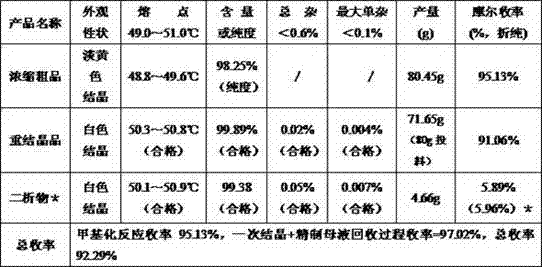
[0055] The reducing agent sodium sulfite in the methylation reaction of Example 1 was discarded, and all other processes and conditions were unchanged. The yield and quality results are shown in Table 2.
[0056] Table 2: Summary of output and quality results of each intermediate product and final product in Example 2
[0057]
[0058] The results of Example 2 suggest that after the reducing agent in the methylation reaction in Example 1 was discarded, the purity and yield of the methylated crude product and primary crystallized product decreased significantly. It needs to undergo secondary recrystallization to basically reach the current quality standard of this product.
Embodiment 3
[0059] Embodiment 3 (the control example that improves reaction pH value)
[0060] During the operation process of the methylation reaction in Example 1, the control range of the pH value of the methylation reaction process was increased to 8.5-10.5, and other reaction conditions remained unchanged. The quality and yield levels of the obtained crude product and primary and secondary refined products are listed in Table 3.
[0061] Table 3: Summary of output and quality levels of intermediate products and final products in Example 3
[0062]
[0063] The results of Example 3 suggest that if the pH range of the methylation reaction in Example 1 is increased to 8.5-10.5, the yield and purity of the methylation reaction will drop significantly, and two refinements are required to basically reach the current quality standard of this product. . Compared with Example 1, the consumption of alkalization and methylation reagent in the reaction process is greatly improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com