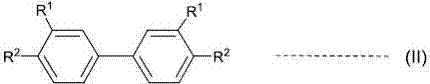
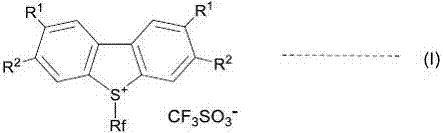
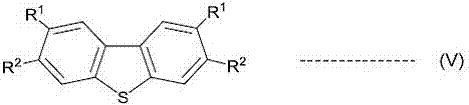
A novel method for preparing S-(perfluoroalkyl)-dibenzothiophenium trifluoromethanesulfonate
A technology of benzothiophene trifluoromethanesulfonate and perfluoroalkylsulfinate, which is applied in the direction of sulfonate preparation, organic compound preparation, chemical instruments and methods, and can solve the problem of trifluoromethanesulfonate Problems such as low thermal stability, high price of trifluoromethanesulfonic anhydride, product purification and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]
[0073] 500ml dry jacketed glass reactor, equipped with condenser, a drying tube and magnetic suspension stirring. 88.7 g (422 mmol) of trifluoroacetic anhydride and 21.8 g (19.1 mmol) of trifluoroacetic acid were added. The temperature of the liquid is controlled by a machine (hot and cold fluid exchange) which further controls the temperature of the reaction mixture in the jacketed reactor. The reaction solution was cooled to -6°C, stirred, and dry sodium trifluoroacetic acid methanesulfonate was added in batches within 7 minutes. After the addition was complete, the temperature of the reaction solution was 1°C, and then stirred at 0°C for 3 hours. Cool to -20°C. Trifluoromethanesulfonic acid (59.0 g, 393 mmol) was added dropwise over 44 min. Stir for 30 minutes, then add 36.5 g (192 mmol) of 3,3'-difluorobiphenyl dropwise at -20°C to 25°C within 75 minutes, after the drop is complete, stir for 16 hours, then heat up to 0°C. 19 F NMR analysis of the reaction s...
Embodiment 2
[0079] Example 2: Preparation and preparation of 3,3'-difluoro-6-(trifluoromethylsulfinyl)biphenyl and 3,3'-difluoro-4-(trifluoromethylsulfinyl)biphenyl characterize
[0080]
[0081]Under nitrogen protection, add sodium trifluoromethanesulfinate (3.9g, 25mmol) and trifluoromethanesulfonic acid (13.2ml, 0.15mol) into a 100ml three-necked flask, stir for 5min, add 3,3'-difluorobiphenyl (4.75g, 25mmol), heated to 60°C and stirred for 4 hours. The reaction solution was mixed with 20ml of water, neutralized with 35ml of saturated sodium carbonate, and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate, filtered, the filtrate was concentrated to dryness, and separated by silica gel column chromatography to obtain 5.59 g (73%) of a yellow oil as 3,3'-difluoro-6-(trifluoromethylsulfinyl) Mixture of biphenyl and 3,3'-difluoro-4-(trifluoromethylsulfinyl)biphenyl (3:1). Further silica gel column chromatography can obtain pure product 3,3'-difluoro-6-(...
Embodiment 3
[0086] Example 3: Confirmation of 3,3'-difluoro-6-(trifluoromethylsulfinyl)biphenyl to obtain 2,8-difluoro-S-(trifluoromethyl)-biphenyl through ring closure reaction Thiophene trifluoromethanesulfonate
[0087]
[0088] Starting material 3,3'-difluoro-6-(trifluoromethylsulfinyl)biphenyl (2.98 g, 9.72 mmol) was 3.5:1 3,3'-difluoro-6-(trifluoromethyl A mixture of sulfinyl)biphenyl and 3,3'-difluoro-4-(trifluoromethylsulfinyl)biphenyl. The starting material was dissolved in 12.5ml of dry nitromethane, cooled to 0-5°C in an ice-water bath, and trifluoromethanesulfonic acid (3.53g12.5mmol) was added dropwise. After stirring overnight at room temperature, the solvent was evaporated to dryness under reduced pressure, and 10 ml of water and 10 ml of toluene were added and stirred for 30 minutes. Filtration, the filter cake was washed with 10ml toluene and 20ml ethyl acetate respectively to obtain 3.45g (81%) of 2,8-difluoro-S-(trifluoromethyl)-dibenzothiophene trifluoromethanesul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com