Method for preparing p-methoxybenzaldehyde perfume in presence of metalloporphyrin through catalytic oxidation of p-methoxytoluene
A technology of p-methoxybenzaldehyde and p-methoxytoluene, which is applied in the field of preparing p-methoxybenzaldehyde fragrance by catalytic oxidation of p-methoxytoluene by metalloporphyrins, can solve the problem of low catalyst activity and selectivity, increased energy Consumption and operational risk, large amount of metal salt catalyst, etc., to achieve the effect of reducing resource consumption and operating costs, easing reaction conditions, reducing energy consumption and operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
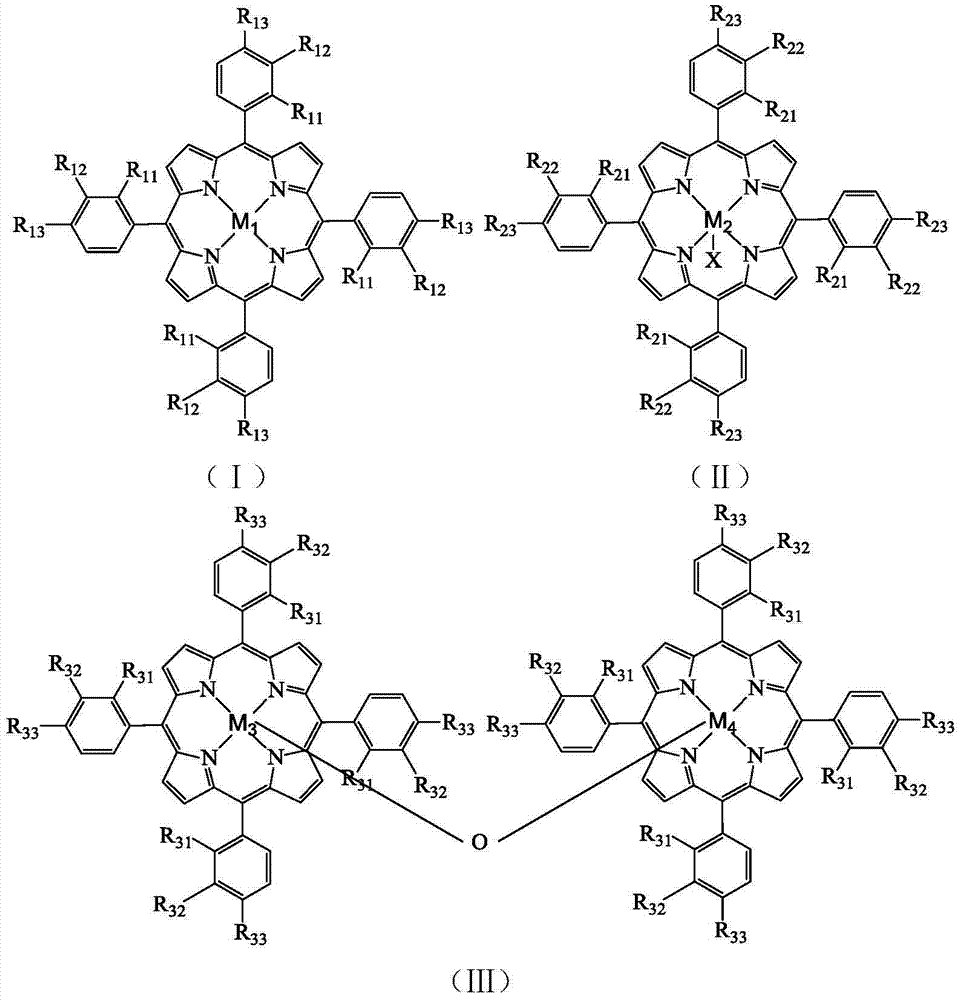
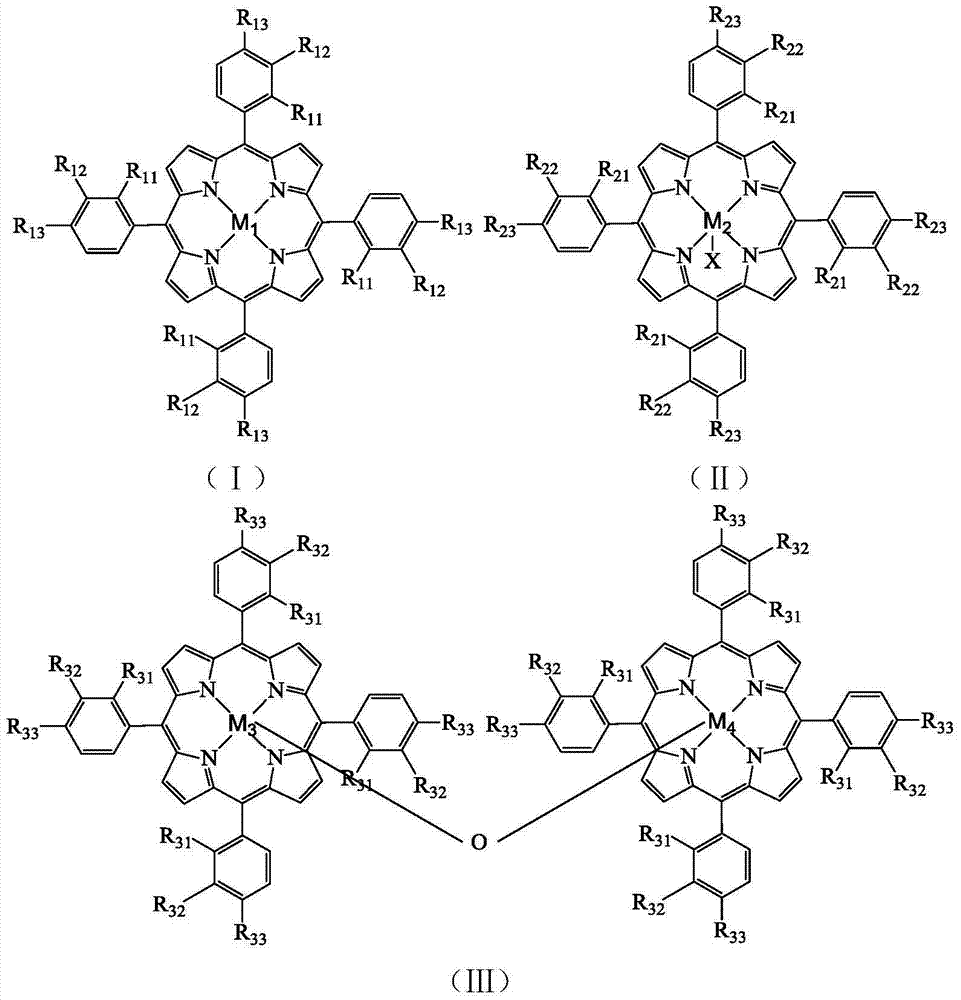
[0028] Take 8.2×10 -4 g tetrakis chloride-(p-methoxyphenyl)iron porphyrin (i.e. R in the formula (II) 21 =R 22 = H, R 23 =OCH 3 , X=Cl,M 2 =Fe), 2.4g of p-methoxytoluene and 0.1619g of N-hydroxyphthalimide were added to a 100mL autoclave, 17.5mL of acetonitrile was added, and oxygen at a pressure of 0.4MPa was introduced, and the temperature was controlled in a water bath for 70 It was reacted at ℃ for 4h, and the reacted mixture was distilled under reduced pressure, and then purified by column chromatography to obtain p-methoxybenzaldehyde. As detected by high performance liquid chromatography, the conversion rate of p-methoxytoluene was 48.5%, the selectivity of p-methoxybenzaldehyde was 46.3%, and the yield of p-methoxybenzaldehyde was 22.5%.
Embodiment 2
[0030] Take 1.7×10 -3 g chloride tetra-(p-nitrophenyl)iron porphyrin (i.e. R in the formula (II) 21 =R 22 = H, R 23 =NO 2 , X=Cl,M 2 =Fe), 2.4g of p-methoxytoluene and 0.1619g of N-hydroxyphthalimide were added to a 100mL autoclave, 17.5mL of acetonitrile was added, and oxygen at a pressure of 0.2MPa was introduced, and the temperature was controlled in a water bath for 70 It was reacted at ℃ for 4h, and the reacted mixture was distilled under reduced pressure, and then purified by column chromatography to obtain p-methoxybenzaldehyde. As detected by high performance liquid chromatography, the conversion rate of p-methoxytoluene was 38.2%, the selectivity of p-methoxybenzaldehyde was 38.7%, and the yield of p-methoxybenzaldehyde was 14.8%.
Embodiment 3
[0032] Take 1.3×10 -3 g tetrakis chloride-(p-methoxyphenyl)iron porphyrin (i.e. R in the formula (II) 21 =R 22 = H, R 23 =Cl,X=Cl,M 2 =Fe), 2.4g of p-methoxytoluene and 0.0971g of N-hydroxyphthalimide were added to a 100mL autoclave, 17.5mL of acetonitrile was added, and oxygen at a pressure of 0.2MPa was introduced, and the temperature was controlled in a water bath at 65 It was reacted at ℃ for 10 h, and the reacted mixture was distilled under reduced pressure, and then purified by column chromatography to obtain p-methoxybenzaldehyde. As detected by high performance liquid chromatography, the conversion rate of p-methoxytoluene was 37.0%, the selectivity of p-methoxybenzaldehyde was 36.2%, and the yield of p-methoxybenzaldehyde was 13.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com