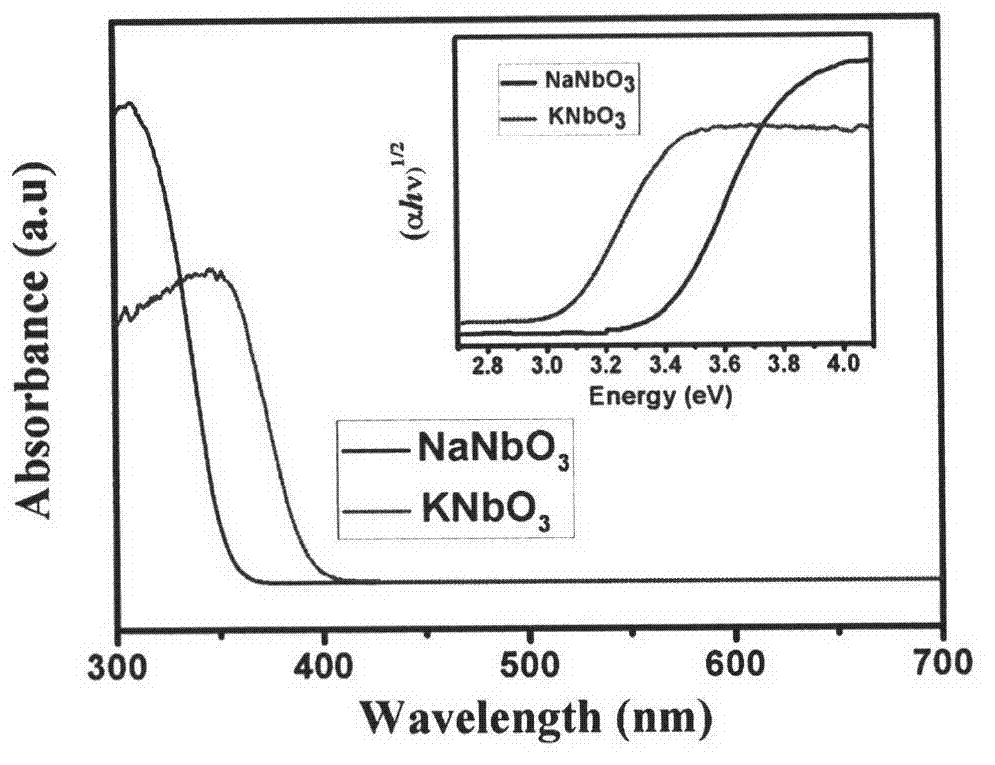
Preparation and applications of alkali metal niobate photocatalytic material
An alkali metal niobate, photocatalytic material technology, applied in metal/metal oxide/metal hydroxide catalysts, hydrocarbon production from carbon oxides, physical/chemical process catalysts, etc., can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] (1) Nb 2 o 5 , Na 2 CO 3 , K 2 CO 3 According to KNbO 3 Stoichiometric mixing.
[0014] (2) After grinding the mixture, preheat it at 800° C. for 4 hours.
[0015] (3) After regrinding, calcining at a temperature of 900° C. for 5 hours.
[0016] (4) Add KNbO to the methanol solution 3 Powder and chloroplatinic acid were irradiated by a 400W high-pressure mercury lamp for 1h.
[0017] (5) After the obtained precipitate was filtered, washed with distilled water and ethanol, and dried in an oven at 70° C. for 12 hours.
[0018] The invention provides a preparation method of an alkali metal niobate photocatalytic material and its photocatalytic reduction of CO 2 performance. Using CH 4 to evaluate the photocatalytic reduction of CO 2 performance. This scheme is further described by the following examples.
specific Embodiment approach 2
[0019] (1) 0.100g of Pt-KNbO 3 The powder sample is evenly dispersed on the bottom of a glass container, and the glass container is placed on the bottom of a high borosilicate glass container (this is a closed container).
[0020] (2) After sealing the reactor, use a gas sampler to inject CO into the reactor 2 After displacing the air therein, 3 mL of water was injected into the reactor with a liquid syringe. The reactor was placed in a dark place for 2 h to allow the system to reach adsorption-desorption equilibrium.
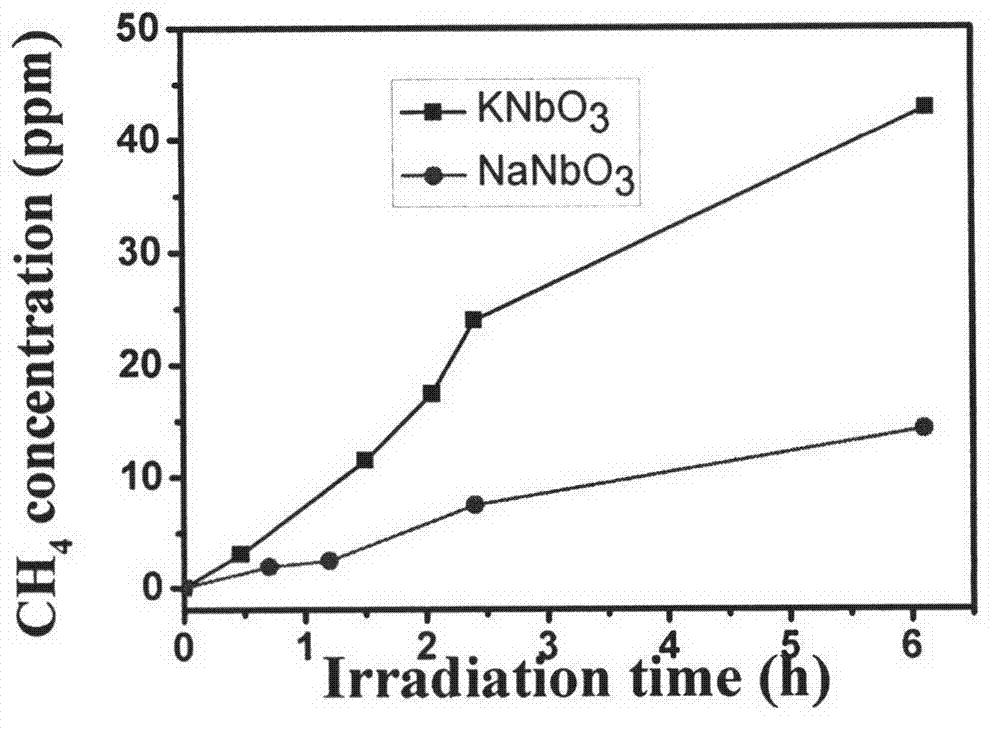
[0021] (3) Place the reactor under a xenon lamp light source to start the reaction, and regularly take samples from the reaction system, and use a gas chromatograph to detect CH 4 concentration.
[0022] After 6h of irradiation, CH 4 The production rate is 7.0ppm / h.
specific Embodiment approach 3
[0023] Different from the second specific embodiment, the photocatalytic material powder used in step (1) is Pt-NaNbO 3 , after 6h of reaction, CH 4 The production rate is 2.3ppm / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com