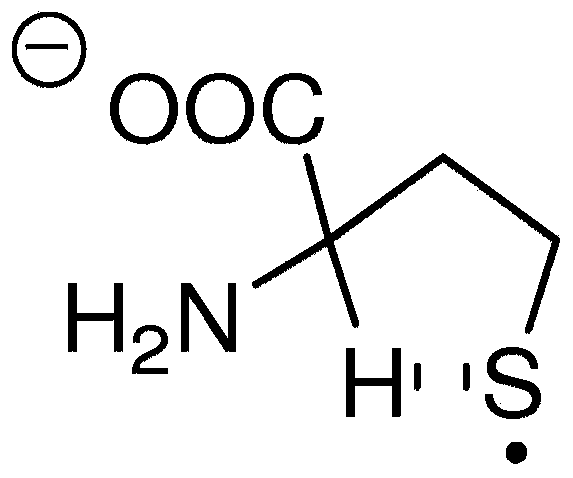
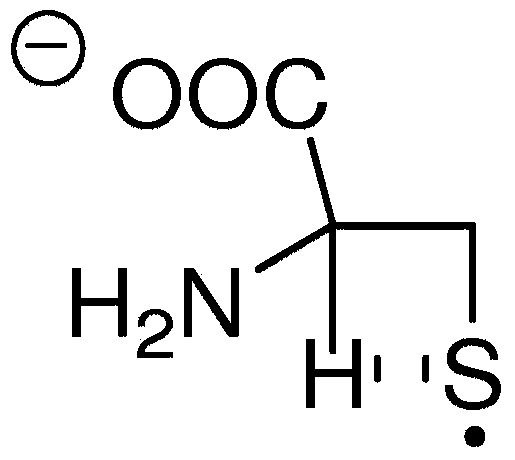
Analyte detection using near-infrared fluorophores
A fluorophore and compound technology, applied in the field of using near-infrared fluorophores to detect analytes, can solve the problem of undisclosed homocysteine colorimetric selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0251] Synthesis and Characterization of Near Infrared Fluorophores
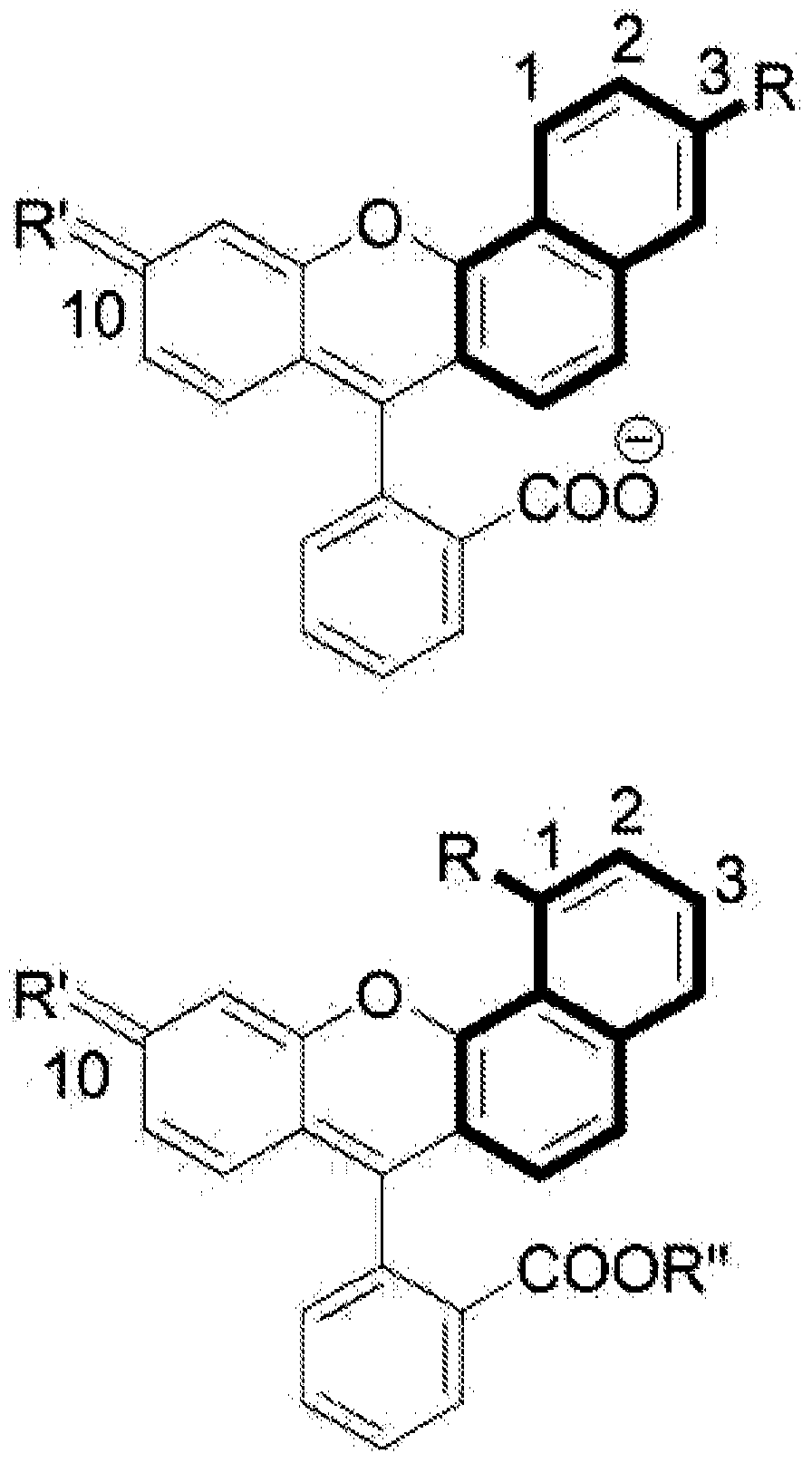
[0252] Such as reaction scheme 1 ( Figure 5 ) by acid condensation of 1,8-dihydroxynaphthalene with phthalic anhydride in methanesulfonic acid or by 1,8-dihydroxynaphthalene with the corresponding aldehyde in 85% H 3 PO 4 Condensation at 125°C / 24h to synthesize analogs with the structure of general formula IV. Naphthofluorescein methyl ester 22 was obtained from 21 via a typical Fischer esterification scheme (Scheme 4). Via hydroxybenzophenone and corresponding naphthol in CH 3 SO 3 Asymmetric semi-naphthofluorescein, rhodol and rhodamine analogues of general formula III were synthesized by acid condensation in a 1:1 mixture of H:TFA at 80°C for 16-24 h (Schemes 2 and 3). The desired hydroxybenzophenone and 1,8-naphthalene derivatives were synthesized following described or modified literature protocols. Methyl ester derivatives were prepared by H in MeOH 2 SO 4 or HCl-catalyzed esterification; by i...
Embodiment 2
[0301] Synthesis of para-, ortho-, and meta-bridged diviologens
[0302] Synthesize para-bridged viologen according to Scheme 13:
[0303] Scheme 13
[0304]
[0305] Bipyridine (16.66 g, 106.65 mmol) was dissolved in 125 mL of acetonitrile, and the solution was refluxed. Next, p-bis(bromomethyl)benzene (5 g, 18.94 mmol) was dissolved in 300 mL of acetonitrile. This solution was added to the refluxing solution of bipyridine over 1 hour. After complete addition of the p-bis(bromomethyl)benzene solution, the mixture was continued to reflux for 24 hours. The formed precipitate was filtered, washed with acetonitrile (2x50 mL) and air dried. Single bridged viologen 103 was obtained as a light yellow solid. Yield 10.5g, 96%. 1 H NMR (DMSO-d 6 ,400MHz): δ(ppm)9.4-9.41(4H,d),8.85-8.86(4H,d),8.65-8.67(4H,d),8.00-8.01(4H,d),7.74(4H,s) ,5.94(4H,s).
[0306] Compound 103 (3 g, 5.3 mmol) was suspended in 30 mL of water, and the mixture was heated until the precipitate was compl...
Embodiment 3
[0323] Emissions in the blood
[0324] Compound 15d (Table 1) was diluted to 40 μM in 50% DMSO:50% 25 mM pH 9 phosphate buffer. Such as Figure 30 As shown in , diluted compounds produce fluorescence whose λ max ex =~690nm and λ max em =~790nm (Stokes shift=~100nm, 1834cm -1 ). Whole blood (pig blood in Na-EDTA, Lampire Biological Laboratories) was included in the aqueous portion of the solvent to yield a final blood concentration of 5% by volume, causing only minimal loss of fluorescence due to hemoglobin absorption and Scattering from blood components. The emission remained stable for at least 1 hour. The absorption curve matches the output of the laser pointer (635-670nm; max output 90% at >750nm), it is possible to NIR emission (80 μM) is clearly visible. Similar results were observed with 5% whole blood, but an increase in scattered excitation light was clearly visible in both the visible and NIR images, which was easily overcome using a second filter.
[0325] H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com