CDDO ethyl ester polymorphic substance and application thereof
A technology of polymorphism and polymorphism, which is applied in the direction of medical preparations containing active ingredients, drug combinations, steroids, etc., and can solve problems such as weak biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Synthesis of the compound of formula I
[0063]
[0064] Step 1 Synthesis of APSN13B-1
[0065] name
M.W.
the amount
mol
equivalent
456
1000g
2.2mol
1
EtI
156
376g
2.4mol
1.1
K 2 CO 3
138
604g
4.4mol
2
DMF
/
12L
/
/
[0066] To a solution of oleanolic acid (1000g, 2.2mol) and potassium carbonate (604g, 4.4mol) dissolved in DMF (12L) was added iodoethane (376g, 2.4mol). The resulting mixture was stirred overnight at 45°C. After oleanolic acid could not be detected by HPLC, the mixture was cooled to room temperature and poured into water (120 L). The resulting suspension was stirred for 30 minutes. The solid was collected by a centrifuge, washed with water (1L) and dried in vacuum at 50°C to obtain 976g APSN13B-1 for subsequent steps. The yield is 92%.
[0067] Step 2. Synthesis of APSN13B-2
[0068] name
M.W.
the amount
mol
equivalent
APSN13B-1
484
975g
2mol
1
Ac 2 O
102
612g
6mol
3
79
474g
6mol
3...
Embodiment 2
[0102] Example 2. Preparation of the crystal form of the compound represented by formula I
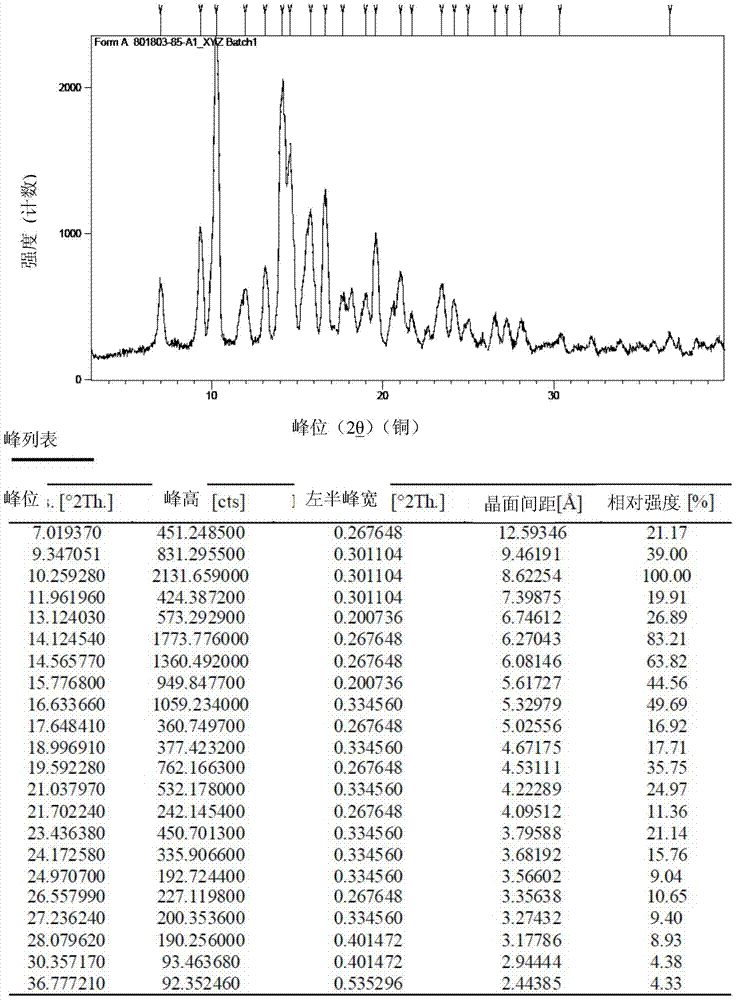
[0103] The compound of formula I prepared by the method described in Example 1 above is dissolved in heptane solvent at room temperature, and then the desired crystal form I is obtained by natural precipitation, and its melting point is 174-177°C.
[0104] figure 1 The X-ray powder diffraction pattern shown in Table 1 is summarized.
[0105] Table 1
[0106]
Embodiment 3
[0107] Example 3. Preparation of the crystal form II of the compound represented by formula I
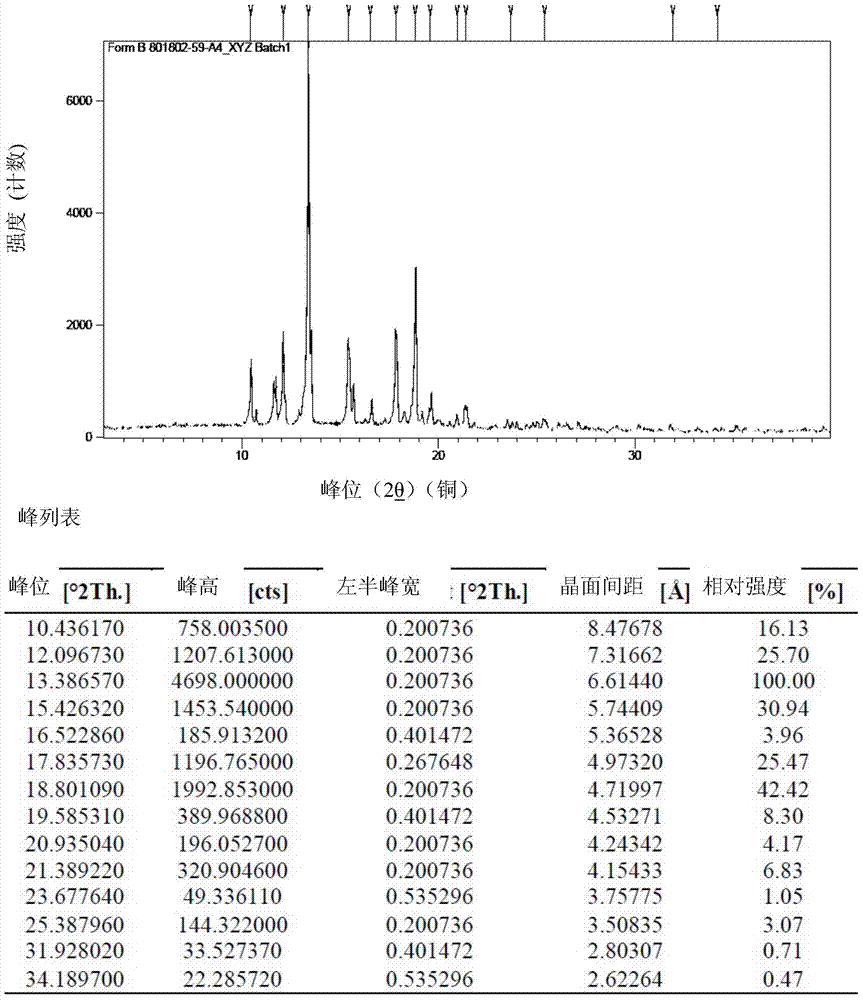
[0108] The compound represented by the formula I prepared by the method described in the above-mentioned embodiment 1, the excess of the compound is added to the mixed solvent of ethyl acetate and heptane (the ratio of ethyl acetate and heptane is 1:10, the weight ratio or volume Compared with), stir to make the slurry, and stir at room temperature or 50°C for at least 48 hours to obtain crystal form II with a melting point of 209-212°C.
[0109] figure 2 The X-ray powder diffraction pattern shown in Table 2 is summarized.
[0110] Table 2
[0111]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com