Cysteine fluorescence probe adopting coumarin as fluorophore and application of cysteine fluorescence probe
A fluorescent probe, cysteine technology, applied in the field of cysteine fluorescent probe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 3-Acetyl-7-hydroxycoumarin is obtained by reacting 2,4-dihydroxybenzaldehyde with ethyl acetoacetate, and then reacted with acryloyl chloride to obtain 2-(3-acetyl)coumarinyl acrylate. The generated 2-(3-acetyl)coumarin-based acrylate can undergo Mike addition cyclization reaction with cysteine to release fluorescent 3-acetyl-7-hydroxycoumarin, thereby realizing detection .
[0024] Above-mentioned reaction process reaction formula is as follows:
[0025]
[0026] Add 2,4-dihydroxybenzaldehyde (1, 20mmol), ethyl acetoacetate (24mmol) and 1mL piperidine into 30mL absolute ethanol, heat to reflux, react for 3 hours and cool to room temperature, a green solid precipitates , filtered with suction, washed with ethanol three times, and the crude product was recrystallized with ethanol to obtain light green solid 2, melting point: 237°C, yield 91%.
[0027] Infrared measurement (KBr, cm -1 ): 3482(O-H), 3050(Ar-H), 2928(=CH), 1678(C=O), 1620(C=C), 1211(C-O-C).
[0028...
Embodiment 2
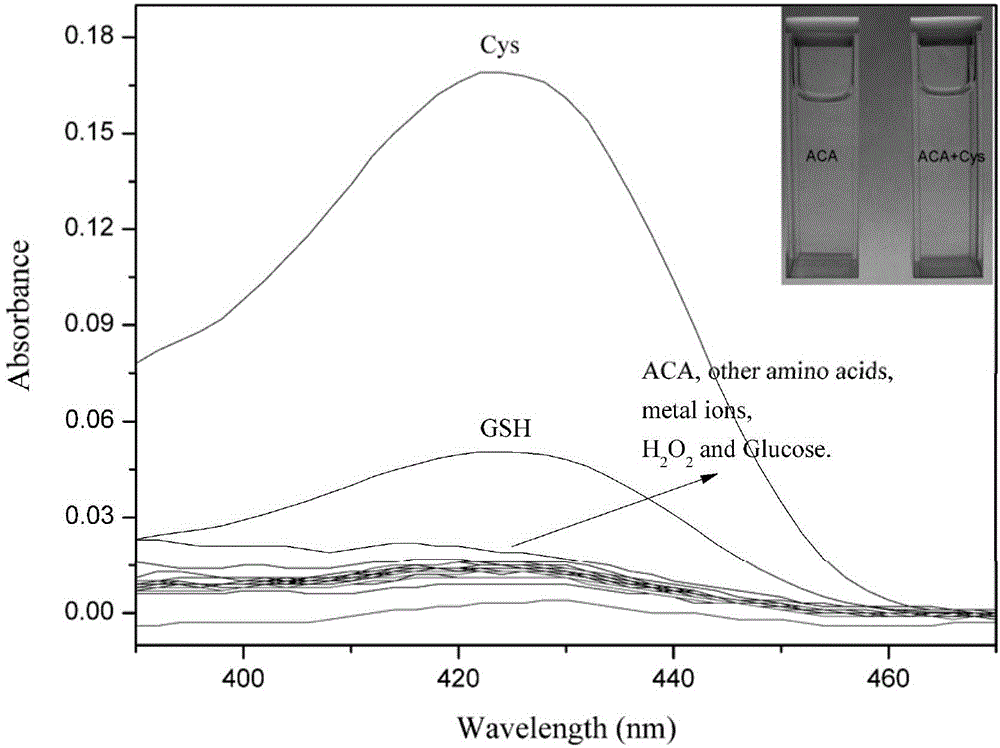
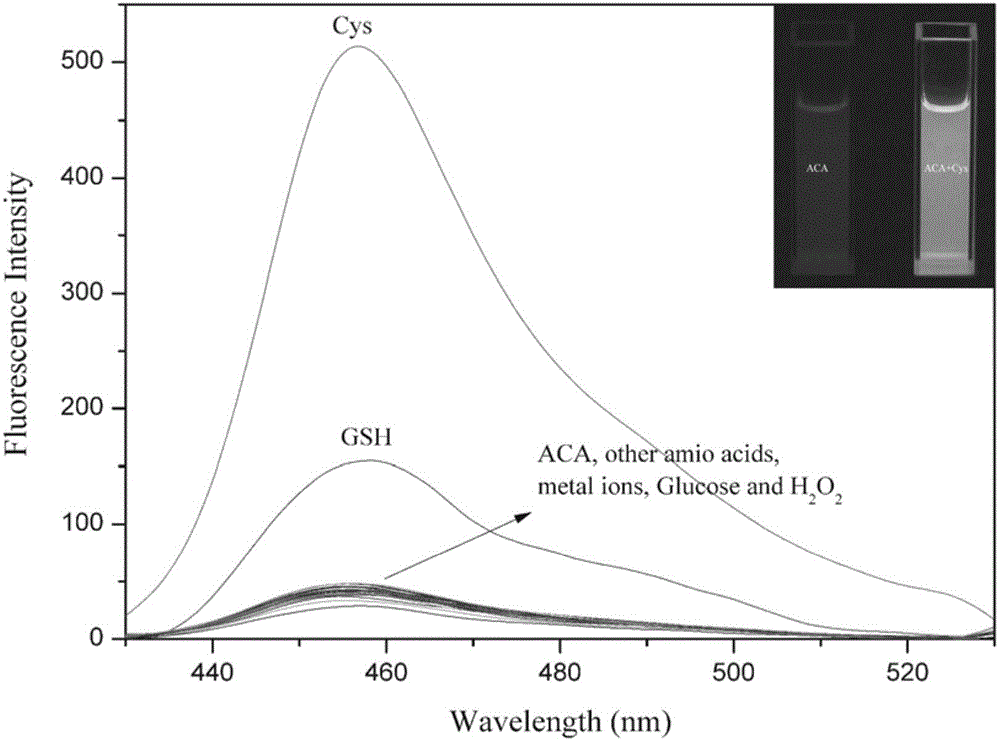
[0037] Add 60 μM of Cys,Hcy,GSH,arginine,aspartic acid,glutamicacid,glycine,histidine,lysine,proline,threonine,tryptophan,tyrosine,KNO 3 , Ca(NO 3 ) 2 ,NaNO 3 ,Mg(NO 3 ) 2 , Zn(NO 3 ) 2 ,Fe(NO 3 ) 3 ,H 2 o 2 And the aqueous solution of glucose, carry out UV-visible spectrophotometry and fluorescence spectrophotometry test after 3 hours, show that probe 2-(3-acetyl) coumarin base acrylate has better selectivity to cysteine , the control before and after adding cysteine showed a strong fluorescence enhancement effect. See figure 1 ,2.
Embodiment 3
[0039] Intracellular fluorescence imaging test:
[0040] The two groups of A and B are the control group, and the control group A: under the condition of 37 ℃, HeLa cells are cultured in the cell culture medium of 20.0 μ M 2-(3-acetyl) coumarin-based acrylate for 5 hours; Group B: HeLa cells were soaked in cell culture medium containing 20mM N-ethylmaleimide for 2 hours, washed three times with PBS buffer solution, and then added 20.0μM 2-(3-acetyl)coumarin Incubate in acrylated cell culture medium for 5 hours. Fluorescence imaging shows the penetration of 2-(3-acetyl)coumarinyl acrylate into the cells. In the control group A cells showed strong fluorescence. Control group B showed no fluorescence. See Figure 4 .
[0041] Fluorescence test in bovine serum:
[0042] At 37°C, 1 μM of 2-(3-acetyl)coumarinyl acrylate was added to different concentrations of bovine serum solutions (0, 0.01, 0.05, 0.2, 0.5, 1) and incubated for 20 minutes. The fluorescence intensity was dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com