A Proportional Fluorescent Probe of Sulfur Dioxide Derivatives Using Semi-Cyanine and Flavonol as Fluorophores and Its Application
A fluorescent probe, sulfur dioxide technology, used in fluorescence/phosphorescence, organic chemistry, luminescent materials, etc., can solve problems such as human health hazards and adverse reactions, and achieve good selectivity, specificity, and high sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 The preparation of the ratio fluorescent probe of sulfur dioxide derivatives using semi-chuancyanine and flavonol as fluorophores according to the present invention
[0024] O-hydroxyacetophenone and p-carboxybenzaldehyde undergo ring closure in two steps to obtain flavonol fluorophores, and then introduce aromatic aldehyde groups through the construction of amide bonds, and then condense with indole salts to obtain compound L-HF1.
[0025] Above-mentioned reaction process reaction formula is as follows:
[0026]
[0027] Compound 1 (1mmol), Compound 2 (1mmol), DMAP (0.1mmol) and EDC (1mmol) were added to 30mL of dichloromethane, and after reacting at room temperature for 8 hours, the organic phase was washed with brine, dried over anhydrous sodium sulfate, and pumped After filtration, the liquid phase was spin-dried, and silica gel column chromatography (dichloromethane: ethyl acetate = 1:1) gave compound 3 with a yield of 82% and a melting point of 238...
Embodiment 2
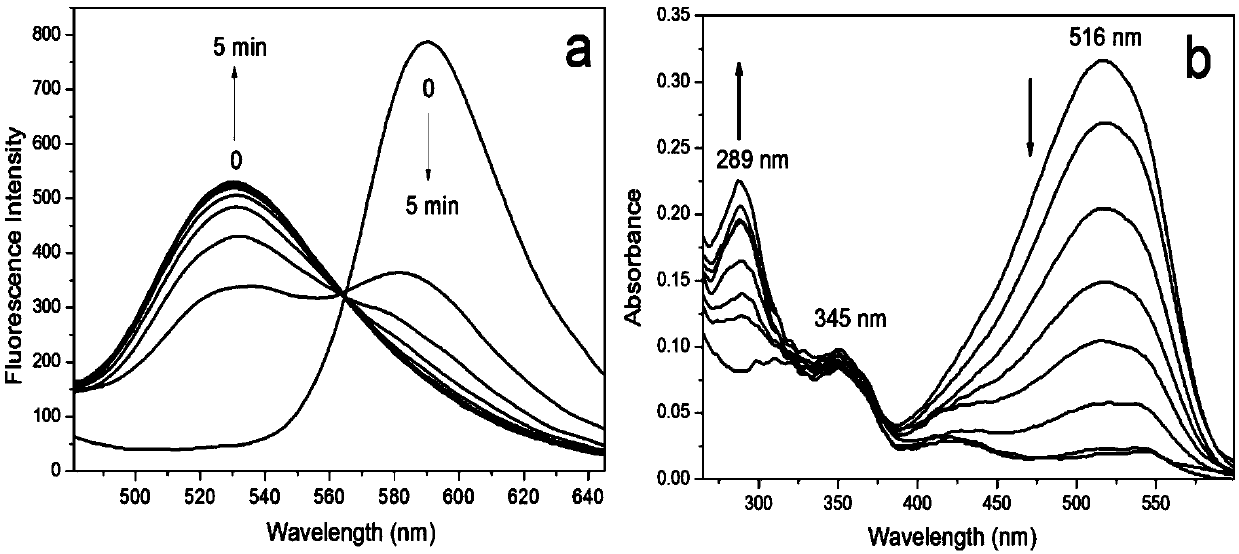
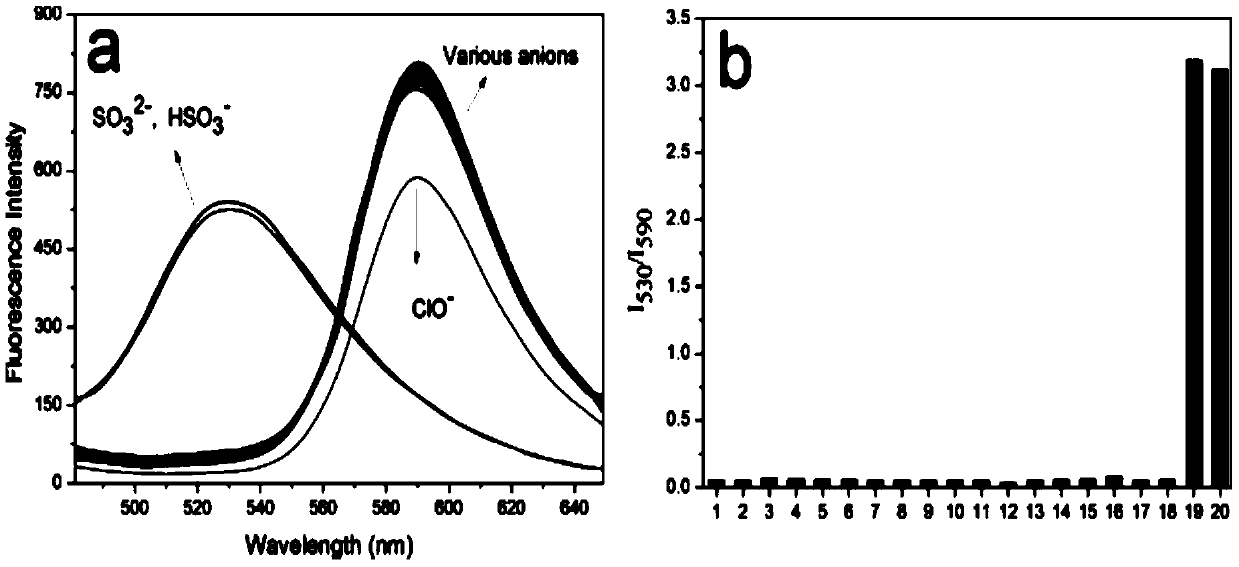
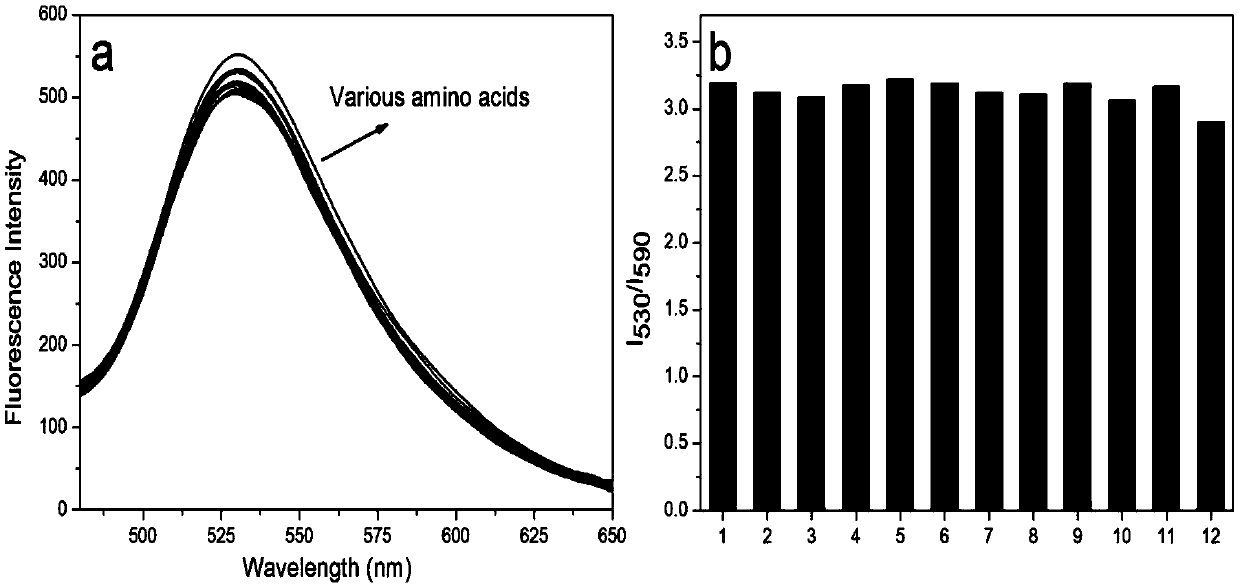
[0038] Using a microsyringe, quantitatively add various anions (F - , Cl - ,Br - , I - , HCO 3 - ,NO 3 - ,NO 2 - ,N 3 - ,AcO - ,H 2 PO 4 - ,ClO - ,ClO 3 - , SO 4 2- , HSO 4 - ,HS - ,SCN - ,S 2 o 3 2- , HSO 3 - and SO 3 2- ) and various amino acid molecules (glutamate, histidine, lysine, tryptophan, valine, arginine, sarcosine, aspartic acid, cysteine, homocysteine, glutathione) at a final concentration of 1 mM, after 5 minutes of action, the fluorescence spectrophotometry test was performed, and the probe It has good selectivity to sodium sulfite and sodium bisulfite, and the comparison before and after adding sodium sulfite and sodium bisulfite shows obvious changes in fluorescence emission. See figure 1 , 2, 3.
Embodiment 3
[0040] Intracellular fluorescence imaging test:
[0041] HeLa cells: at 37°C, HeLa cells were cultured for 1 hour in the cell culture solution added with 5 μM L-HF1; then cultured in the cell culture solution with different concentrations of sodium bisulfite for 0.5 hours. Fluorescence imaging showed that the probe L-HF1 could penetrate into the cells, and the intensity ratio of green fluorescence to red fluorescence gradually increased with the increase of sodium bisulfite concentration. See Figure 4 .
[0042] L-02 cells: at 37°C, L-02 cells were cultured for 1 hour in the cell culture solution added with 5 μM L-HF1, and then cultured in the cell culture solution with different concentrations of sodium bisulfite for 0.5 hours. Fluorescence imaging showed that the probe L-HF1 could penetrate into the cells, and the intensity ratio of green fluorescence to red fluorescence gradually increased with the increase of sodium bisulfite concentration. Add 5 μM probe L-HF1 to live...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com